Manuscript accepted on : 18-04-2025
Published online on: 22-05-2025
Plagiarism Check: Yes
Reviewed by: Dr. Vinutha K B
Second Review by: Dr. Asmare Amuamuta Limeneh
Final Approval by: Dr. Hifzur Siddique
Gopalakrishnan Rajesh 1 , Ramachandran Ragunathan1*
, Ramachandran Ragunathan1* and Jesteena Johney2
and Jesteena Johney2
1Department of Biotechnology, Centre for Bioscience and Nanoscience Research, Bharathiar University, Coimbatore, Tamilnadu, India
2Department of Food and Nutrition, Centre for Bioscience and Nanoscience Research, Bharathiar University, Coimbatore, Tamilnadu, India.
Corresponding Author E-mail:cbnrcinidia@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3395
ABSTRACT: This study aimed to extract γ-aminobutyric acid (GABA) from Lactiplantibacillus plantarum isolated from milk. The microorganism was identified through standard microbiological techniques, DNA isolation, polymerase chain reaction (PCR), and gene sequencing analysis, followed by database submission to NCBI, obtaining the accession number PP391551. GABA extraction was performed using ethyl acetate in MRS broth, and its presence was confirmed through Fourier-transform infrared spectroscopy (FTIR), thin-layer chromatography (TLC), and high-performance liquid chromatography (HPLC) and obtained yield of 1.8 g/L. The extracted GABA exhibited antibacterial activity against Shigella dysenteriae (21±0.044 mm), Salmonella typhi (22±0.08 mm), Escherichia coli (11±0.12 mm), and Klebsiella pneumoniae (13±0.48 mm), as well as antifungal activity against Aspergillus niger (14±0.072 mm) and Aspergillus flavus (11±0.061 mm). Additionally, cytotoxicity analysis against the HCT 116 cell line revealed an IC₅₀ value of 103.48 µg/ml, highlighting its potential biomedical applications.
KEYWORDS: Antimicrobial; Cytotoxicity; FTIR; GABA; HPLC; Lactiplantibacillus plantarum; TLC
Download this article as:| Copy the following to cite this article: Rajesh G, Ragunathan R, Johney J. Assessment of Antimicrobial and Cytotoxicity effect of GABA extracted from Lactiplantibacillus plantarum. Biotech Res Asia 2025;22(2). |
| Copy the following to cite this URL: Rajesh G, Ragunathan R, Johney J. Assessment of Antimicrobial and Cytotoxicity effect of GABA extracted from Lactiplantibacillus plantarum. Biotech Res Asia 2025;22(2). Available from: https://bit.ly/4jkxS6v |
Introduction
GABA, also known as gamma-aminobutyric acid (GABA), is a non-protein amino acid produced through the glutamate decarboxylation pathway. GABA serves as an antidepressant, antihypertensive, antidiabetic, antimicrobial, anticancerous and immune system booster while also benefiting neural health. It is present in various foods, including grains, vegetables, fruits, 1 and animal products, as well as in different organisms such as bacteria, cyanobacteria, fungi, algae, and plants. 2
Over the years few researches has been undergone to find the most suitable strategies to increase the amount of GABA in food with plant enrichment, chemical synthesis and microbial fermentation. Microbiological production of GABA is safer and more environmentally friendly, high specificity, and cost effectiveness than chemical methods. Additionally, using microorganisms for production allows for better control of conditions compared to extraction from plants and animals. Given its pharmaceutical properties, it is essential to establish optimal conditions for GABA production. 3,4,5 GABA production by beneficial microorganism has potential to increase the functional effect of some foods and beverages.4 Lactic acid bacteria are often naturally present in some traditional food fermentations and which is used as starters in few industrial food fermentations for the pro technological properties.6 For the fortification of food like GABA-fortified foods, high amount of GABA producing lactic acid bacteria are being used recently. 7 Lactic acid bacteria have the intrinsic property and numerous strains are proposed as human and animal probiotics. 8
GABA producing lactic acid bacteria strains belonging to the genera of Pediococcus, Lactobacillus, Enterococcus, Lactococcus, Streptococcus, Weisella, Lacticaseibacillus, Secundilactobacillus, Leuconostoc etc. 9,10 Among this Lactobacillus planatrum is facultative hetero fermentative species with high adaptability to many conditions, which already isolated from cereals, vegetables, bee bread, meat, milk, fruits and fermented foods.11 L.plantarum is a normal inhabitant of the gastro-intestinal tract insects, mammals, fish and which includes in qualified presumption of safety and in generally recognized as safe lists.12
In recent years, researchers have investigated Lactobacillus spp., particularly for its ability to synthesize GABA. However, comprehensive bioactivity evaluations remain limited. The present study aimed to isolate a unique strain of Lactiplantibacillus plantarum from milk, extract and characterize GABA, and conduct a detailed bioactivity evaluation. This included an antimicrobial activity study against foodborne pathogens and an anticancer study against the HCT 116 cell line.
Materials and Methods
Collection of sample and isolation of Lactobacillus
The milk sample was collected from the cow farm of Eachanari, Coimbatore, TamilNadu, India in a sterile container and processed. MRS broth (Himedia, Mumbai, India) was prepared by dissolving 55.15g in 1000ml of distilled water and sterilsed under autoclave at 1210C for 15minutes with 15lbs. The sterilized media was cooled to room temperature and the collected milk sample was added (10ml broth and 0.5ml milk) and incubated at 370C for 24 to 48 hours for the enumeration and isolation of the lactic acid bacteria.
Isolation of pure culture from the broth one loop of the bacteria was used to streak on MRS plate and incubated at 370C upto 24 hours. After incubation single colony was re streaked on to MRS agar and incubated which was repeated for 3 to 5 time to get the pure Lactobacillus spp. and was used for further study. 10
Molecular confirmation of L.plantarum
Isolated bacterial DNA was extracted with phenol chloroform method 13 and 16s ribosomal RNA sequence was amplified using the universal forward primer 27 F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and reverse primer 1492R (5′-CTA CGG CTA CCT TGT TAC GA-3′) by AB Applied Biosystem PCR. The PCR cycling condition were ; initial denaturation at 950C/5 min, 30 cycles of denaturation at 950C/30 sec, annealing at 55 0C/20 sec, and extension at 720 C/5 min, and a final extension at 72o C/5 min. The amplified gene product was subjected to sequencing analysis and the obtained data was compared using the Basic Local Alignment Search Tool (BLAST) available at the NCBI (National Center for Biotechnology Information, USA) followed by the phylogenetic tree was constructed.14
Production and Characterization of GABA – TLC, FTIR and HPLC
MRS broth was used as the production medium. After inoculation, the medium was incubated at 37°C for 5 to 7 days, followed by centrifugation at 10,000 rpm for 10 minutes. The supernatant was then precipitated using a 60% ethyl acetate solution. Intermediate mixing was carried out for 3 hours, and the mixture was incubated under ice-cold conditions (4°C) for 24 hours. The aqueous layer was then separated and stored at 40C in a sealed amber vial and used for analysis. 15
Initially TLC study was carried out with standard GABA using Rf value to confirm the presence of the compound in the sample. About 10 µL of both standard GABA and the extracted GABA were loaded onto a TLC sheet coated with silica, then placed in a TLC chamber containing an N-butanol, acetic acid, and water mixture in a 5:3:2 ratio. Once the solvent reached approximately 1 cm from the top of the sheet, it was removed and allowed to dry. 0.2% of the ninhydrin solution was sprayed on the sheet and dried to get the visibility of the compounds and the Rf value was calculated.16 FTIR study was carried out for the extracted and standard GABA using Shimadzu infrared spectrophotometer from 4000 to 400 cm-1 to identify the functional group in the GABA.15 A High-Performance Liquid Chromatography (HPLC) study was conducted for both standard and extracted GABA following the specified protocol of 17,18. The samples were injected in the HPLC system equipped with a C18 column (250 X 4.6mm, Shimadzu) and photo diode Array detector. The mobile phase used was methanol and trifluoro acetic acid in the ratio of 60:0.1 with the flow rate of 2 ml/min with 254nm. The presence of GABA was confirmed with standard curve obtained in different concentration of standard GABA.
Antimicrobial study
For the antibacterial study, nutrient agar plate (28g in 1000ml of distilled water followed by sterilized) was used. 24hours old culture of Escherichia coli, Salmonella typhi, Klebseilla pneumoniae and Shigella dysentriae were swabbed (70µl) aseptically swabbed on to the plates. Wells were created with cork borer, and samples were added along with positive control (chloramphenicol-C30mcg) and negative control (DMSO). The plates were then incubated at 370C for 24hrs. After incubation the zone of inhibition (in mm) was measured using antibiotic zone scale (Himedia) in triplicates.
Antifungal study – malt agar (in 1000ml of distilled water followed by sterilized) was seeded with 70µl of Aspergillus niger and Aspergillus flavus. The test samples were added to the wells following previously mentioned method. Fluconazole (1mg/ml) was used as the positive control. The plates were then incubated at 30°C for 5 days, and the zone of inhibition was measured.19
Cytotoxicity study against HCT 116 Cell Line
Human colon cancer cell line (HCT 116 Cell Line) was purchased from NCCS, Pune, India and sub cultured in DMEM medium (Himedia, India) with the addition of 10% FBS, 1 mM L-glutamine and 1% penicillin to avoid the contamination. Cells were cultured in a humidified incubator at 370C with 5% CO2 saturation for 72hours. Viability of the cells were confirmed with MTT assay by adding 100µl of cell line in 96 well plate with different concentration (12.5, 25, 50, 100, 200µg) of sample along with control (untreated cell line) and standard (doxorubicin-1uM/ml) for 24 hours. After incubation the cells were washed with trypsin and DMSO (50µl each) followed by adding MTT dye and incubated for 4 hours. Viability of the cells was measured using ELISA reader at 570nm and the percentage of viability was calculated in triplicates.20
Data analysis
The experimental data were analyzed in triplicates and expressed as mean ± standard deviation (SD). The results were visually represented using graphs for better clarity and comprehension
Results
Molecular confirmation of the bacteria
Molecular identification of the isolated bacteria was performed with Polymerase Chain Reaction (Figure 1) to amplify the 16SrDNA. Alignment of the amplified product of 16SrDNA gene from Gen bank database resulted in the identification of Lactobacillus spp as Lactiplantibacillus plantarum with 100 % homology and the accession number was PP391551 (Figure 2).
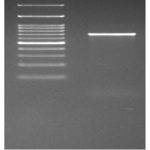 |
Figure 1: PCR amplification of the isolated bacteria. |
 |
Figure 2. Phylogram |
Production and Characterization of GABA – TLC, FTIR and HPLC
The presence of GABA was identified using a TLC study, the Rf value was recorded as 0.63, which was similar to that of standard GABA (Figure 3).
 |
Figure 3: TLC profile of GABA (1- Extracted GABA; 2- Standard GABA). |
The FTIR spectrum of the extracted GABA was compared with standard GABA and 12 functional groups were observed as similar in both sample, which are Peaks at 2985.81 denoted the presence of CH weak, 1735.93 is the presence of C=O ester strong, 1373.32 – CH3 bend, 1234.44 C-O-C, 1095.57 and 1041.56 denotes the presence of C-OH stretch, 848.68 correspond to C-F bond, 786.96 – C-Cl strong, 632.65, 609.51 is corresponding to strong C-Br, 501.49 is denoting the presence of C-I strong bond respectively. FTIR results were presented in Figure 4 and 5.
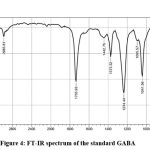 |
Figure 4: FT-IR spectrum of the standard GABA. |
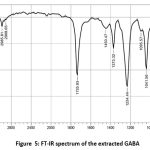 |
Figure 5: FT-IR spectrum of the extracted GABA |
Further, the presence of extracted GABA was confirmed and quantified using HPLC study (Fig. 6), which revealed that L.planatarum can produce 1.8 g/L of GABA. Similarly other researchers also quantified the GABA with HPLC study and the amount may be varying based upon the organism and medium condition.
 |
Figure 6: HPLC chromatogram |
Antimicrobial study
In the agar diffusion assay, the antibacterial activity of L.plantarum was evaluated using the cell free supernatant (CFS) and purified compound (GABA) against gram-negative bacteria such as Escherichia coli, Klebsiella pneumoniae, Salmonella typhi, and Shigella dysentriae. The zone of inhibition was examined around the well after 24 hours of incubation at 370 C. For GABA extract from L.plantarum, the highest zone of inhibition was observed against S.dysentriae (21±0.044), and S.typhi (22±0.08) and the lowest zone of bacterial inhibition was observed against E. coli (11±0.12) and K.pneumoniae (13±0.48). Similarly in case of CFS the highest zone of inhibition was observed against K.pneumoniae (9±0.02), and S. typhi (15±0.01) respectively. No visible inhibition was examined for CFS sample against E. colli and S.dysentriae. Reference antibiotic (chloramphenicol-C30mcg) showed marked zone of inhibition against test organism. There is no inhibitory zone for DMSO which serves as negative control (Table 1).
Table 1: Antibacterial activity of the extracted GABA from L.planatrum against different bacteria
| S.No | Name of the organism | Zone of Inhibition (mm ± mean SD) | |||
| CFS | GABA | DMSO | Disc chloramphenicol | ||
| 1 | E. coli | Nil | 11±0.12 | Nil | 6±0.06 |
| 2 | S. typhi | 15±0.01 | 22±0.08 | Nil | 16±0.02 |
| 3 | K. pneumoniae | 9±0.02 | 13±0.48 | Nil | 17±0.07 |
| 4 | S.dysentriae | Nil | 21±0.044 | Nil | 13±0.01 |
Gamma-aminobutyric acid (GABA) produced by Lactobacillus strains has shown promising antifungal activity against Aspergillus niger and Aspergillus flavus. Studies suggest that GABA can disrupt fungal growth by altering membrane integrity, inhibiting spore germination, and interfering with metabolic pathways essential for fungal survival. This bioactive compound offers a natural and potentially safe alternative for controlling fungal contamination in food and agricultural products (Table 2).
Table 2: Antifungal activity of GABA from L.planatrum
| S.No | Name of the organism | Zone of Inhibition (mm ± mean SD) | |||
| Crude | GABA | DMSO | F-fluconazole | ||
| 1 | A.niger | 8±0.022 | 14±0.072 | Nil | Nil |
| 2 | A. flavus | 8±0.043 | 11±0.061 | Nil | Nil |
Cytotoxicity study against HCT 116 Cells
The cytotoxic effect of GABA from L.plantarum on the HCT 116 cell line was evaluated using MTT assay. After incubation, cell viability of the sample was observed to be 96.03, 75.73, 53.74, 37.85 and 28.04 % at concentrations of 12.5, 25, 50, 100, 200µg respectively. In comparison, the standard drug doxorubicin exhibited a cell viability of 38.05%. The IC50 value for 24 hours in HCT116 cells was calculated to be 103.48μg/ml, which suggests that Lactobacillus-derived GABA has moderate anticancer activity. The image acquired with the inverted microscope (Fig. 8) showed that the treated cells with an IC50 value of GABA extract were wrinkled, and as time passed, cell rupture and fragmentation were detected. The MTT assay results revealed that the cytotoxic effect of the investigated GABA was dose- and time-dependent, with a decrease in cell survival as the parameters were increased. A comparison of the 24-hour IC50 value for HCT116 cells to the results of other investigations demonstrates that the extracted GABA compound could have considerable cytotoxic effects (Fig 7).
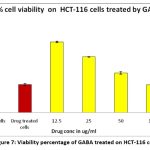 |
Figure 7: Viability percentage of GABA treated on HCT-116 cells |
 |
Figure 8: Effect of GABA against HCT 116 Cells |
Discussion
This study focuses on the isolation of Lactiplantibacillus plantarum from milk, its molecular identification, and the subsequent extraction of gamma-aminobutyric acid (GABA), highlighting the potential application as antimicrobial and anticancer activity.
Initially, Lactiplantibacillus plantarum was successfully isolated, molecularly confirmed, and submitted to the NCBI GenBank, in reference to the work published by Duyen et al, 21 the selected Lactiplantibacillus plantarum possesses 95.70% similarities in the 16SrDNA study and the submission to NCBI Gene Bank. Fifty lactic acid bacteria from the Iranian dairy products were screened for the GABA production and the isolates shows >94 % of similarities and which were considered as belongs to same strains. GABA production may significantly vary between isolates with high similarity and low similarity.22
Phong et al, 23 isolated 12 Lactobacillus strains from Nem Chua samples and fermented them in MRS broth with 1% MSG. The results are consistent with,24 who isolated Lactiplantibacillus plantarum and Levilactobacillus brevis from the fermented bamboo shoot and in the TLC similar spots was observed as standard GABA.
Many researchers studied the compound characterization using FTIR and the peaks are varying based upon the nature of extraction and origin of the extraction. Similarly Lee et al, 25 isolated Lactobacillus sp from Korean salted and fermented sea foods for effective fermentation of strawberry leaf extract to enhance its anti-inflammatory activity and the raw and fermented extracts were subjected to FTIR analysis for the detection of functional groups. In contrast to the extract, different peaks were detected such as peaks at 3218 and 1358 cm−1 are indicators of OH, peaks at 2958 and 2924 cm−1 corresponds to –CH group. 1595 and 1043 cm−1 could be attributed to the stretching of C=C and C–O groups, respectively. 26
An antimicrobial compound was isolated from Lactobacillus delbrueckii subsp. lactis and characterized using FTIR, which revealed peaks ranging from 3227.4 to 1260 cm⁻¹, corresponding to different functional groups such as C=N, NH, and OH.27 GABA from the mulberry leaf shows peaks at around 3379, 2925, 2851, 1738, 1650, 1550, 1247, 1084, and 768 cm-1 and no new chemical group was produced, the particle size were similar. Peak at 3379cm-1 corresponds to intermolecular hydrogen bond OH. 28
Zhang et al ,29 reported that L. planatrum was produced 1.52±0.07g/L. Valenzuela et al 30 stated L.brevis has the ability to produce GABA and which consistent with other reported level of L. brevis LMG9606 (0.29g/L). Hwang et al, 31 examined GABA production with MRS broth with L.lactic strain and found that 1.37g/L of the GABA after 40h of incubation.
Iman et al, 32 found that, E.coli, P.aeruginosa, S. typhi, S.aureus, and E.faecalis exhibited zone of inhibition values of 16mm, 14mm, 19mm, 18mm, and 11mm respectively when tested against Lactobacillus using the agar well diffusion method. The supernatant showed the highest inhibitory zone against S.typhi growth at 19mm, while the least inhibitory zone was observed against E. faecalis at 11mm.
Tanim et al, 33 stated that the antimicrobial activity of the two lactic acid bacteria was primarily assessed using the agar diffusion assay. The cell-free supernatant (CFS) was used to test the inhibitory effects against seven Gram-negative bacteria (A. baumannii, E. coli, K.pneumoniae, P.aeruginosa, S.abony, S.typhi, S.flexneri) and three Gram-positive bacteria (B. cereus, B. subtilis, S. aureus). The CFS of both isolates exhibited a broad antimicrobial spectrum, indicating the presence of inhibitory activity. The L. fermentum strain demonstrated the ability to inhibit all test strains, with a minimum inhibition zone of 9 mm observed against most pathogens. On the other hand, the L. brevis strain displayed a similar antimicrobial spectrum, albeit with relatively smaller inhibition zones (<6 mm) and a minor zone against A. baumannii. Notably, both isolates exhibited the highest effectiveness against P. aeruginosa in the diffusion assay.
Isolation of L. plantarum MYSN7 from a traditional fermented food, haria against fungi shows good antifungal effect against T.tonsurans. 34 The antifungal activity of the GABA against the postharvest pathogen A.alternata as a controlling post harvest disease in fruits and vegetables, these findings confirmed the information about the antifungal activity of the GABA. 35 In another study, the best antifungal agent was screened from 60 isolated Lactobacillus species, revealing that many Lactobacillus strains exhibit antifungal activity against molds and yeasts, including Aspergillus sp., Alternaria sp., Geotrichum sp., Mucor, and Fusarium sp. However, the level of antifungal activity varied, ranging from moderate to poor, depending on the specific compounds extracted. 36
The GABA compound can elicit morphological changes that indicate programmed cell death. Probiotics, particularly lactobacilli, have been studied for their anticancer effects on colorectal cancer cells. L. acidophilus extract and supernatant reduced colorectal cancer cell proliferation and increased apoptosis and necrosis. 37 Another study found that L. acidophilus causes apoptosis in cervical, gastric, breast, and colorectal cancer cells. 38
Combining L. acidophilus and L. casei extracts can cause apoptosis in colorectal cancer cells. 39 A different study on Lactobacillus revealed that external polysaccharides reduce BCL-2 and survivin gene expression while increasing Caspase-3 (Cas3), Caspase-9 (Cas9), and BAX gene expression in colorectal cancer cells, ultimately inducing cell death. 40
The anticancer activity of CaCo-2 and HT 29 cells using Lactobacillus culture free supernatant showed increasing cytotoxicity at the concentration of 800 µL/mL. Similarly, the growth of HT-29 cells was inhibited in a dose-dependent manner following treatment with CFSs. The inhibitory rates were comparable to those of 2.5 μM 5-fluorouracil (5-FU), a positive control known to inhibit approximately 50% of HT-29 cells. 41 These discussions suggests that, Lactobacillus plantarum components in the GABA compound may promote colorectal cancer cell death by regulating apoptosis-related gene expression. This preliminary study focused on the anticancer effects of GABA from Lactobacillus plantarum on HCT 116 cells. While normal cell comparisons were not included, Lactobacillus plantarum is a probiotic known for its non-toxic nature, suggesting no impact on healthy cells.
Conclusion
This study demonstrated the successful isolation of Lactiplantibacillus plantarum from milk using MRS broth and the production of γ-aminobutyric acid (GABA), which was precipitated using an ethyl acetate solution. The extracted GABA was characterized using Fourier Transform Infrared Spectroscopy (FTIR) and confirmed through Thin-Layer Chromatography (TLC) and High-Performance Liquid Chromatography (HPLC), yielding 1.8 g/L. Antimicrobial studies revealed significant antibacterial activity against Escherichia coli, Salmonella typhi, Klebsiella pneumoniae, and Shigella dysenteriae, as well as antifungal activity against Aspergillus niger and Aspergillus flavus. Additionally, the cytotoxicity study demonstrated effective toxicity against the HCT 116 cell line, with an IC50 value of 103.48 µg/mL. This study presents a novel approach for extracting and confirming GABA from L. plantarum, highlighting its potential as a natural antimicrobial and anticancer agent.
Acknowledgement
The authors express heartfelt thanks to the management and staffs of Centre for Bioscience and Nanoscience Research, Eachanari, Coimbatore-21, Tamilnadu, India, for giving the facility to complete the research work.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement-
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Permission to reproduce material from other sources
Not Applicable
Author Contributions
Gopalakrishnan. Rajesh – Research work and Writing,
Ramachandran Ragunathan-Conceptualization and Reviewing
Jesteena Johney- Supervision, Reviewing and Editing
References
- Dhakal R, Bajpai VK, Baek, KHm. Production of GABA (γ-aminobutyric acid) by microorganisms: A review. Brazilian J Micro. 2012; 43:1230–41.
CrossRef - Sunita A Ramesh, Stephen D Tyerman, Matthew Gilliham, Bo Xu, γ-Aminobutyric acid (GABA) signalling in plants. Cell Mol Life Sci. 2017; 74(9): 1577–03.
CrossRef - Bai Q, Yang R, Zhang L, Gu Z. Salt stress induces accumulation of γ–aminobutyric acid in germinated foxtail millet (Setaria italica). Cereal Chem. 2013; 90: 145–49.
CrossRef - Sahab NRM, Subroto E, Balia RL, Utama GL γ-Aminobutyric acid found in fermented foods and beverages. 2020; 6(11): e05526.
CrossRef - Luo H, Liu Z, Xie F, Bilal M, Liu L, Yang R, Wang Z. Microbial production of gamma-aminobutyric acid: Applications, state-of-the-art achievements, and future perspectives. Crit Rev Biotech. 2021; 41(4): 491–12.
CrossRef - Wang Y, Wu J, Lv M, Shao Z, Hungwe M, Wang J, Bai X, Xie J, Wang Y, Geng W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front Bio Eng Biotech. 2021; 9:
CrossRef - Diana M, Quilez J, Rafecas M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J Fun Foods. 2014; 10: 407–20.
CrossRef - Behera SS, Ray RC, Zdolec N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. Biomed Res Int. 2018; 9361614.
CrossRef - Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Micro. 2012; 113(2): 411–17.
CrossRef - Devi PB, Rajapuram DR, Jayamanohar J, Verma M, Kavitake D, Meenachi Avany BA, Rani PU, Ravi R, P.H. Gamma-aminobutyric acid (GABA) production by potential probiotic strains of indigenous fermented foods origin and RSM based production optimization. LWT. 2023; 176: 114511.
CrossRef - Testa B, Lombardi SJ, Tremonte P, Succi M, Tipaldi L, Pannella G, Sorrentino E, Iorizzo, M, Coppola R. Biodiversity of Lactobacillus plantarum from traditional Italian wines. World J Micro Biotech. 2014; 30(8): 2299–05.
CrossRef - Koutsoumanis K, Allende A, Alvarez-Ordonez A, Bolton D, Bover-Cid S, Chemaly M, Lieve, Herman. Update of the list of qualified presumption of safety (QPS) recommended microbiological agents intentionally added to food or feed as notified to EFSA. EFSA J. 2022; 21 (1): 1-23.
CrossRef - Heuer H, Krsek M, Baker P, Smalla K, Wellington E M. Analysis of Actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Env Micro. 1997; 63: 3233–41.
CrossRef - Abirami N, Jesteena J, Ragunathan R. Production of surfactin from novel Bacillus isolated from soil and its antifungal properties. J Env Bio. 2023; 44: 728-35.
CrossRef - Long Zheng, Xuli Lu, Shengtao Yang, Ying Zou, Fanke Zeng, Shaohao Xiong, Yupo Cao, Wei Zhou. The anti-inflammatory activity of GABA-enriched Moringa oleifera leaves produced by fermentation with Lactobacillus plantarum. Front Nutri. 2023; 10:1093036.
CrossRef - Huynh Xuan Phong, Le Quoc Viet, Luu Minh Chau, Bui Hoang Dang Long, Nguyen Ngoc Thanh, Dao Tan Phat, Le Dang Truong. Isolation and Selection of Lactic Acid Bacteria with the Capacity of Producing γ-aminobutyric Acid (GABA) and Antimicrobial Activity: Its Application in Fermented Meat Product. Cur Nutr Food Sci. 2023; 19: 831-37.
CrossRef - Le PH, Verscheure L, Le TT, Verheust Y, Raes K. Implementation of HPLC analysis of γ-aminobutyric acid (GABA) in fermented food matrices. Food Anal Met. 2020; 13: 1190–01.
CrossRef - Palanisamy Bruntha Devi, Dileep Reddy Rajapuram, JabastinJayamanohar, Manika Verma, Digambar Kavitake, Bargavi A, Meenachi Avany, Potunuru Uma Rani, Ramasamy Ravi, PrathapkumarHalady Shetty. Gamma-aminobutyric acid (GABA) production by potential probiotic strains of indigenous fermented foods origin and RSM based production optimization. LWT- Food Sci Tech. 2023; 176: 114511.
CrossRef - Jesteena Johney, S Radhai Sri, R Ragunathan. Extraction of Chitin and Chitosan from Wild Type Pleurotus spp and its Potential Application – Innovative Approach. J Pure Appl Micro. 2018; 12(3): 1631-40.
CrossRef - Jesteena Johney, S Radhai Sri, R Ragunathan. Development of Chitosan Silver Nanocomposites: Its Characteristic Study and Toxicity Effect against 3T3-L1 Cell Line. J Pure Appl Micro. 2022; 16(1): 494-02.
CrossRef - Duyen TH, Binh NNH, Viet LQ, Chau LM, Thanh NN, Phat DT, Truong LD, Phong HX. Selection and identification of antibacterial and GABA producing lactic acid bacteria from Vietnamese fermented meat. The 6th International Conference on Eco Engineering Development. 2022; 1169.012095.
CrossRef - Mohammad Reza, EdalatianDovom, Mohammad Bagher Habibi Najaf, Paria Rahnama Vosough, Neda Norouzi, Seyyed Javad Ebadi Nezhad, Baltasar Mayo. Screening of lactic acid bacteria strains isolated from Iranian traditional dairy products for GABA production and optimization by response surface methodology. Sci Rep. 2023; 13: 440.
CrossRef - Huynh Xuan Phong, Le Quoc Viet, Luu Minh Chau, Bui Hoang Dang Long, Nguyen Ngoc Thanh, Dao Tan Phat, Le Dang Truong. Isolation and Selection of Lactic Acid Bacteria with the Capacity of Producing γ-aminobutyric Acid (GABA) and Antimicrobial Activity: Its Application in Fermented Meat Product. Cur Nutr Food Sci. 2023; 19: 831-37.
CrossRef - Meilin Chen, Hongqiu Xia, Xifeng Zuo, Danping Tang, Haoyu Zhou, Zijun Huang, Ailing Guo, Jun Lv. Screening and characterization of lactic acid bacteria and fermentation of gamma-aminobutyric acid-enriched bamboo shoots. Front Micro. 2024; 15: 1333538.
CrossRef - Se-Won Lee, Ui-Lim Choi, Jeong-Muk Lim, Seong-Hyeon Lee, Harshavardhan Mohan, Kamala-Kannan Seralathan, Yool-Jin Park, Byung-Taek Oh. Isolation of Lactiplantibacillus from Korean salted and fermented sea foods for effective fermentation of strawberry leaf extract: enhanced anti-inflammatory activity. 3 Biotech. 2021; 11(6): 268.
CrossRef - Savic D, Jokovic N, Topisirovic L. Multivariate statistical methods for discrimination of Lactobacilli based on their FTIR spectra. Dairy Sci Tech. 2008; 88(3): 273–90.
CrossRef - Narendrakumar Gopakumaran, Sri Gajani Veerasangili1, Preethi Thozhikatu Valliaparambal, Isolation and Characterization of Bacteriocins like Antimicrobial Compound from Lactobacillus delbrueckii subsp lactis. Jordan J Bio Sci. 2017; 10(4): 221-27.
- Yingchun Jin, Jie Tu, Xinyao Han, Jun Zhuo, Guanhui Liu, Yanhui Han, Hengjun Du, Jun Wang, Hang Xiao. Characteristics of Mulberry Leaf Powder Enriched With γ-Aminobutyric Acid and Its Antioxidant Capacity as a Potential Functional Food Ingredient. Front Nutr. 2022; 9: 900718.
CrossRef - Zhang Q, Zeng L, Tan X, Tang J, Xiang W. An efficient γ-aminobutyric acid (GABA) producing and nitrite reducing ability of Lactobacillus plantarum BC114 isolated from Chinese paocai. Food Sci and Tech. 2017; 23(5): 749–55.
CrossRef - Valenzuela JA, Florez AB, Vazquez L., Vasek OM, Mayo B. Production of γ-aminobutyric acid (GABA) by lactic acid bacteria strains isolated from traditional, starter-free dairy products made of raw milk. Ben Micro. 2019; 10(5): 579–87.
CrossRef - Hwang E, Park JY. Isolation and characterization of gamma-aminobutyric acid (GABA)-producing lactic acid bacteria from Kimchi. Cur Top L A B & Pro. 2020; 6(2): 64–69.
CrossRef - Dehghani Champiri Iman, Bamzadeh Zahra, Rahimi Ebrahim, Rouhi Leila. Isolation and Identification of Lactobacillus brevis from Cottage Cheese of Bazoft City, Iran and Evaluation of Its Antimicrobial Activity Against Some Pathogenic Microorganisms. Iran J Med Micro. 2022; 16: 17-34.
CrossRef - Hossain Tanim, Mozumder Halima, Ali Ferdausi, Akther Khadiza, Inhibition of pathogenic microbes by the lactic acid bacteria Limosilactobacillus fermentum strain LAB-1 and Levilactobacillus brevis strain LAB-5 isolated from the dairy beverage borhani. Cur Res Food Nutr Sci. 2022 10(3): 928-39.
CrossRef - Vanitha PR, Somashekaraiah R, Divyashree S, Pan I, Sreenivasa MY. Antifungal activity of probiotic strain Lactiplantibacillus plantarum MYSN7 against Trichophyton tonsurans. Front Micro. 2023; 14: 1192449.
CrossRef - Sainan Yu, Chaoying Zhen, Pengyu Zhao, Junjie Li, Zhen, Qin, Haiyan Gao. Antifungal mechanisms of γ-aminobutyric acid against the postharvest pathogen Alternaria alternate. LWT – Food Sci Tech. 2023; 173: 114314.
CrossRef - Lidia Lipinska, Robert Klewicki, Elzbieta Klewicka, Krzysztof Kolodziejczyk, Michal Sojka, Adriana Nowak. Antifungal Activity of Lactobacillus Bacteria in the Presence of Xylitol and Galactosyl-Xylitol. Bio Med Res Int. 2016; 8: 5897486.
CrossRef - Dallal MMS, Mojarrad M, Baghbani F, Raoofian R, Mardaneh J, Salehipour Z. Effects of probiotic Lactobacillus acidophilus and Lactobacillus casei on colorectal tumor cells activity (CaCo-2). Archives of Iranian medicine. 2015; 18(3): 167-72.
- Nami Y, Abdullah N, Haghshenas B, Radiah D, Rosli R, Khosroushahi AY. Probiotic potential and biotherapeutic effects of newly isolated vaginal Lactobacillus acidophilus 36YL strain on cancer cells. Anaerobe. 2014; 28: 29-36.
CrossRef - Baldwin C, Millette M, Oth D, Ruiz MT, Luquet FM, Lacroix M. Probiotic Lactobacillus acidophilus and casei mix sensitize colorectal tumoral cells to 5-fluorouracil-induced apoptosis. Nutri cancer. 2010; 62(3): 371-78.
CrossRef - Tukenme U, Aktas B, Aslim B, Yavuz S. The relationship between the structural characteristics of lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci Rep. 2019; 9(1): 8268.
CrossRef - Yuan-Chunm Yue, Bao-Yu Yang, Jing Lu, Shu-Wen Zhang, Liu Liu, Khaled Nassar, Xiao-Xi Xu, Xiao-Yang Pang, Jia-Ping Lv. Metabolite secretions of Lactobacillus plantarumYYC-3 may inhibit colon cancer cell metastasis by suppressing theVEGF-MMP2/9 signaling pathway. Microbial Cell Fact. 2020; 19(213): 1-12.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





