Manuscript accepted on : 06-09-2025
Published online on: 24-09-2025
Plagiarism Check: Yes
Reviewed by: Dr. Maysloon Hamied
Second Review by: Dr. Makhabbah Jamilatun
Final Approval by: Dr. Wagih Ghannam
Farwa Jabeen , Marta Martini
, Marta Martini and Paolo Ermacora*
and Paolo Ermacora*
Department of Agricultural Science and Biotechnology, University of Udine, Udine, Italy.
Corresponding Author Email: paolo.ermacora@uniud.it
ABSTRACT: Kiwifruit is a nutritious fruit, but often its quality and shelf life decline due to fungal diseases. Cadophora luteo-olivacea, known for causing skin-pitting, is a as a key threat to postharvest storage of kiwifruit. Through both in vitro and in vivo tests, this study aimed to determine the the antifungal ability of volatile organic compounds (VOCs) produced by Bacillus pumilus QST2808 for controlling this postharvest pathogen fungal pathogen. In vitro results showed 52% reduction in fungal growth when exposed to these VOCs for 14 days. In storage trials, kiwifruits treated with VOCs showed a 28.5% decrease in disease severity after 96 hours of exposure compared to the untreated control, followed by three months in cold storage. However, this reduction was not statistically significant (p > 0.05). These findings highlight the potential of B. pumilus QST2808 VOCs as an eco-friendly biofumigation approach to manage C. luteo-olivacea-induced postharvest skin-pitting disease in kiwifruit.
KEYWORDS: Biofumigation; Biological control; Bacillus pumilus; Kiwifruit; Postharvest
| Copy the following to cite this article: Jabeen F, Martini M, Ermacora P. Suppression of Postharvest Skin-Pitting Disease in Kiwifruit by Volatile Organic Compounds (VOCs) from Bacillus pumilus QST2808. Biotech Res Asia 2025;22(3). |
| Copy the following to cite this URL: Jabeen F, Martini M, Ermacora P. Suppression of Postharvest Skin-Pitting Disease in Kiwifruit by Volatile Organic Compounds (VOCs) from Bacillus pumilus QST2808. Biotech Res Asia 2025;22(3). Available from: https://bit.ly/47UusW1 |
Introduction
Kiwifruit (Actinidia deliciosa) is famous for its flavor and nutritional value10 and can be stored for up to five months under normal refrigeration (0 °C and 92%–95% relative humidity).1 However, during postharvest handling, these fruits become vulnerable to fungal infections, particularly through injuries sustained during harvesting and processing. Among the pathogens responsible for substantial postharvest losses, Cadophora luteo-olivacea—the causal agent of skin-pitting disease has recently become a serious postharvest problem in Italian packaging companies.4 This fungus infects the fruit during development and remains dormant until symptoms appear during prolonged cold storage (typically 3–4 months), causing substantial economic challenges for the kiwifruit industry.3
Biological control methods offer a sustainable alternative to chemical fungicides, especially given concerns about chemical residues, environmental impact, and the emergence of resistant pathogens.9 Among the various mechanisms of actions used by antagonistic microbes, the emission of antifungal volatile organic compounds (VOCs) has gained attention for their antifungal properties, although this strategy is still relatively underexplored (Spadaro & Droby).7
Several biological control agents (BCAs), including bacterial species from the genera Pseudomonas and Bacillus, are known to produce volatile compounds capable of antifungal properties.8 Previous research demonstrated that P. synxantha VOCs showed a considerable reduction in kiwifruit infections caused by C. luteo-olivacea and Botrytis cinerea, highlighting its potential as a biological control agent.2 However, Bacillus pumilus have gained increasing attention due to their ability to produce a wide spectrum of antifungal metabolites but, little is known about the effectiveness of VOCs from B. pumilus against C. luteo-olivacea, especially under realistic storage conditions.
Therefore, the present study explores the antifungal action of VOCs emitted by B. pumilus QST2808, both in laboratory settings and during cold storage against C. luteo-olivacea. Additionally, its performance is compared with the well-known VOC-producing strain P. synxantha 1172b. This research aimed to contribute to integrated postharvest disease control strategies using bacterial VOCs in kiwifruit storage.
Materials and Methods
Fruits and Microorganisms
In Friuli Venezia Giulia (FVG), Italy, the commercially ripe kiwifruit cultivar “Hayward” [Actinidia deliciosa (A. Chev)] were harvested from an orchard. Prior to the experiment, only fruits that were uniform in size and free of obvious lesions were chosen, and they were kept at 0°C and 92% relative humidity for five days.
At the University of Udine-Di4A, the fungal strain C. luteo-olivacea (Cad21) was isolated from infected kiwifruit tissue and molecularly identified. Before the experiment, fungal cultures were maintained at 25°C for two weeks on potato dextrose agar (PDA; 39 g L⁻¹, Oxoid, UK).
An active component of the biocontrol product Sonata®, the bacterial strain B. pumilus QST2808, was acquired from the Northern Regional Research Laboratory (NRRL), located in Illinois, USA. Bacterial strains were cultivated on nutrient agar (NA; 13 g L⁻¹, Oxoid, UK) at 25°C. Bacterial cultures were grown and maintained on nutrient agar (NA; 13 g/L, Oxoid, UK) at 25 °C. To achieve a concentration of 1×10⁸ cells/mL, a two-day-old culture was prepared in potassium phosphate buffer (PPB); which was made with 70 mL of 0.2 M KH₂PO₄, 30 mL of 0.2 M K₂HPO₄, and 300 mL deionized water; the pH adjusted to 6.5) to reach a concentration of 1×10⁸ cells/mL.
In-vitro assay
A double Petri dish method was used to assessed the antifungal activity of volatile organic compounds (VOCs) generated by B. pumilus QST2808 against the mycelial development of C. luteo-olivacea (Cad21) by using the protocol of Di Francesco et al. (2023) with slight modifications. B. pumilus QST2808 bacterial suspension (1 × 10⁸cells mL⁻¹) was equally distributed on nutritional agar (NA) plates. While P. synxantha 1172b (1 × 10⁸ cells mL⁻¹) served as the positive control. Mycelial plugs (6 mm in diameter) of C. luteo-olivacea were extracted from 14-day-old cultures and placed in the middle of potato dextrose agar (PDA; 39 g L⁻¹, Oxoid, UK) plates. The NA plate with bacterial growth was inverted over the PDA plate containing the fungal plug, and both plates were sealed with Parafilm® to form a double-plate system. The negative control consisted of plates treated with 100 µL of sterile distilled water (SDW) on NA. All plates were incubated at 25 °C in darkness for 14 days. The experiment was carried out twice independently, with five replications of each treatment.
In-vivo assay
An in-vivo biofumigation carried out to evaluate the potential and role of volatile organic compounds (VOCs) generated by B. pumilus QST2808 to improve the kiwifruit resistance to skin-pitting during cold storage. The bottom of sterile plastic boxes measuring 29 × 18 × 10 cm (L × W × H) was filled with 150 mL of nutrient agar (NA). After solidification, the agar surface was evenly covered with 600 µL aliquot of a B. pumilus QST2808 solution (1×10⁸ cells mL⁻¹). The boxes were then sealed with Parafilm® and incubated for 48 hours at 25 °C to allow for the buildup of volatile organic compounds.
During this period, kiwifruits were rinsed with distilled water, allowed to air dry, and then disinfected using 0.1% (v/v) sodium hypochlorite for one minute. A sterile nail was used to make wound at the equator (2 × 2 × 2 mm) of the fruit, to inoculate each fruit with 20 µL of C. luteo-olivacea (Cad21) conidial suspension (1×10⁵ conidia mL⁻¹). To prevent direct contact with the medium, the fruits were placed on sterile grids inside the containers once the inoculum had dried. After that, the containers were incubated for 96 hours at 15 °C and 85% relative humidity. Subsequently, the fruits were moved to cold storage at 0 °C for a period of three months. Each treatment group consisted of three containers, with eight kiwifruits in each.
P.synxantha 1172b was used as the positive control for the biofumigation treatment, while control treatments consisted of containers containing NA without bacterial inoculation.
Statistical analysis
Minitab 17 (Minitab Inc., State College, PA, USA) was used to analyse all experimental data using one-way analysis of variance (ANOVA). Tukey’s Honest Significant Difference (HSD) test was used to compare the means of fungal colony diameter and disease severity at a significance level of α = 0.05. The mean ± standard error is used to present the results.
Results
In-vitro assay
The double-plate assay confirmed that the volatile organic compounds (VOCs) released by B. pumilus QST2808 effectively suppressed the growth of C. luteo-olivacea (Cad21). As shown in Figure 1, exposure to these VOCs led to a 52% reduction in fungal colony diameter compared to the untreated control. Similarly, P. synxantha 1172b, used as a positive control, demonstrated a 56% inhibition in mycelial expansion relative to its respective control.
In-vivo assay
The in vivo biofumigation assay further reinforced the antifungal efficacy of B. pumilus VOCs under realistic postharvest storage conditions. As shown in Figure 2, after 96 hours of VOC exposure and subsequent cold storage at 0 °C for three months, fruits treated with B. pumilus VOCs exhibited a 28.5% reduction in skin-pitting severity compared to the untreated control. Similarly, VOCs from P. synxantha 1172b resulted in a 32% reduction in disease severity.
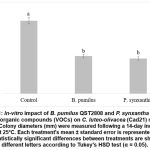 |
Figure 1: In-vitro impact of B. pumilus QST2808 and P. synxantha 1172b’s volatile organic compounds (VOCs) on C. luteo-olivacea (Cad21) mycelial growth. Colony diameters (mm) were measured following a 14-day incubation period at 25°C.
|
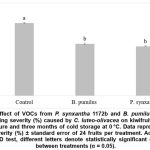 |
Figure 2: Effect of VOCs from P. synxantha 1172b and B. pumilus QST2808 on skin-pitting severity (%) caused by C. luteo-olivacea on kiwifruit after 96 h VOC exposure and three months of cold storage at 0 °C.
|
Data represent mean disease severity (%) ± standard error of 24 fruits per treatment. According to Tukey’s HSD test, different letters denote statistically significant differences between treatments (α = 0.05).
Discussion
The results from both in-vitro and in-vivo studies highlighted how effective the VOCs compounds produced by B. pumilus QST2808. The reduction in fungal colony diameter in the in-vitro setup suggests that VOCs interfere with fungal metabolism and inhibit hyphal growth without direct contact. These outcomes are in line with the earlier research showing that the VOCs produced from B. pumilus have antifungal properties. For example, Morita et al discovered that B. pumilus TM-R produced antifungal volatile organic compounds (VOCs) like ethanol, 5-methyl-2-heptanone, methyl isobutyl ketone, and S-2-methylbutylamine, which inhibited the growth of Penicillium italicum and other fungal infections.6 The inhibitory effect observed in our study suggests that B. pumilus QST2808 may produce similar compounds, which interfere with fungal metabolism and inhibit hyphal growth, thus contributing to pathogen suppression without physical contact.
The in-vivo biofumigation results further confirm the practical potential of using B. pumilus QST2808 VOCs in real postharvest storage environments. Although the reduction in disease severity was not statistically significant, indicating variability under natural storage conditions and the consistent downward trend suggests that VOCs may contribute to disease suppression or induce resistance mechanisms in the fruit. These outcomes are consistent with the findings of Yuan et al’s research , which showed that VOCs from Bacillus velezensis P2-1 reduced postharvest decay in apples caused by Botryosphaeria dothidea.11 The effectiveness of VOCs in these studies reinforces their potential as safe, residue-free alternatives to synthetic fungicides.
Interestingly, the comparable efficacy observed between B. pumilus QST2808 and P. synxantha 1172b underscores the broader applicability of bacterial VOCs in managing kiwifruit postharvest diseases. The results of this study are supported by earlier research by Di Francesco et al which also demonstrated the role of P. synxantha VOCs in suppressing C. luteo-olivacea and B. cinerea.2
Taken together, the results from both in vitro and in vivo assays confirm that VOCs produced by B. pumilus QST2808 possess antifungal activity against C. luteo-olivacea, making them promising candidates for biofumigation in integrated postharvest disease management strategies. Future investigations should aim to identify and characterize the specific VOCs responsible for antifungal activity, optimize exposure conditions, and evaluating their effects on fruit quality and shelf life under commercial conditions.
Conclusion
The study’s conclusions demonstrate the antifungal properties of the volatile organic compounds (VOCs) produced by B. pumilus QST2808 in mitigating the symptoms of skin pitting in kiwifruit that are brought on by C. luteo-olivacea. The effectiveness observed in both in vitro and in vivo assays indicates its suitability as a biofumigation strategy for postharvest disease management. However further investigation is required to optimize treatment conditions, assess consistency across storage environments, and evaluate potential for commercial application.
Acknowledgment
The authors would like to thank the University of Udine for providing technical support.
Funding Sources
This work was supported by Convenzione ERSA for the project “Sviluppo e adattamento del kiwi al cambiamento climatico e alle sindromi emergenti” (CUP: F23C23000300002).
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Permission to reproduce material from other sources
Not Applicable
Author Contributions
Farwa Jabeen: Conceptualization, Methodology, Investigation, Data Analysis, Writing – Original Draft.
Marta Martini: Supervision, Writing – Review & Editing.
Paolo Ermacora: Supervision, Project Administration, Writing – Review & Editing, Correspondence.
References
- Di Francesco A, Jabeen F, Di Foggia M, et al. Study of the efficacy of bacterial antagonists against Cadophora luteo-olivacea of kiwifruit. Biol Control. 2023;180:105199.
CrossRef - Di Francesco A, Jabeen F, Vall-Llaura N, et al. Pseudomonas synxantha volatile organic compounds: efficacy against Cadophora luteo-olivacea and Botrytis cinerea of kiwifruit. Front Plant Sci. 2024;15:1398014.
CrossRef - Jabeen F. Biocontrol Strategies for Effective Kiwifruit Post-Harvest Disease Management. 2024.
CrossRef - Jabeen F, Di Francesco A, Sadallah A, Ermacora P, Martini M. Biocontrol strategies in the management of Cadophora luteo-olivacea, skin-pitting agent of kiwifruit. In: VI International Symposium on Postharvest Pathology: Innovation and Advanced Technologies for Managing Postharvest Pathogens; 2022:75-80.
CrossRef - Jiang Q, Zhang M, Xu B. Application of ultrasonic technology in post-harvested fruits and vegetables storage: a review. Ultrason Sonochem. 2020;69:105261.
CrossRef - Morita T, Tanaka I, Ryuda N, Ikari M, Ueno D, Someya T. Antifungal spectrum characterization and identification of strong volatile organic compounds produced by Bacillus pumilus TM-R. Heliyon. 2019;5(6):e01965.
CrossRef - Spadaro D, Droby S. Development of biocontrol products for postharvest diseases of fruit: the importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci Technol. 2016;47:39-49.
CrossRef - Sukmana AS, Nurcahyanti SD, Nurdika AAH. Biological control of bacterial wilt (Ralstonia pseudosolanacearum) in tomato (Solanum lycopersicum) using an avirulent strain, Bacillus, and Pseudomonas fluorescens. Arch Phytopathol Plant Prot. 2025;58(4):202-222.
CrossRef - Tsalgatidou PC, Papageorgiou A, Boutsika A, et al. Insights into the interaction between the biocontrol agent Bacillus amyloliquefaciens QST 713, the pathogen Monilinia fructicola and peach fruit. Agronomy. 2024;14(4):771.
CrossRef - Wang H, Zhang X, Li K, et al. Glycerol treatment enhances resistance to soft rot disease and maintains postharvest quality in kiwifruit. Postharvest Biol Technol. 2025;228:113630.
CrossRef - Yuan H, Wang L, Hou H, et al. Antifungal activity and mechanism of volatile organic compounds produced by Bacillus velezensis strain P2-1 against Botryosphaeria dothidea-induced postharvest decay in apples. Postharvest Biol Technol. 2025;222:113405.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





