Manuscript accepted on : 25-08-2025
Published online on: 17-09-2025
Plagiarism Check: Yes
Reviewed by: Dr. Gandham Rajeev
Second Review by: Dr. Vivek Anand
Final Approval by: Dr. Eugene A. Silow
S. N. Pradhan Centre for Neurosciences, University of Calcutta, Kolkata, West Bengal, India.
Corresponding Author E-mail: joybiswasofficial97@gmail.com
ABSTRACT: It has been five years since the first COVID-19 cases emerged in China. The infection spread extensively around the world, impacting millions and presenting a significant risk to public health, leading to the breakdown of healthcare systems in various countries. The COVID-19 is caused by the SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) and is a recent emergent virus that belongs to the beta (β)-coronaviruses. The presence of comorbidities significantly influenced the mortality rate among patients infected with COVID-19. For a while, it was believed that the transmission of SARS-CoV-2 was limited to respiratory tract invasion; however, more research indicated that the infection may also affect the central and peripheral neurological systems, among many other organs and systems. Encephalopathy, encephalitis, meningitis, ischemic and hemorrhagic stroke, and cerebral venous sinus thrombosis are among the neurological side effects linked to the SARS-CoV-2 infection. Since the pandemic began, COVID-19 treatment has advanced swiftly, mostly emphasizing antiviral and immunomodulatory medications. Remdesivir and nirmatrelvir-ritonavir are examples of antivirals that have been observed to be most effective when used initially in an illness (for instance, as outpatient treatment) and in instances of milder disease. When treating serious disease or critical illness, immunomodulatory treatments like dexamethasone, Janus kinase inhibitors, or interleukin-6 are most beneficial. Mass vaccination campaigns as a preventative measure are the most effective means of preventing this pandemic. In order to mitigate or lower the rates of COVID-19 infections, hospitalizations, and fatalities, several vaccine platforms, such as vaccines based on nucleic acids (DNA and mRNA vaccines), adenovirus-vectored vaccines, inactivated vaccines, and protein-based subunit vaccines, have been created and developed. This article mainly emphasizes the taxonomy and structural virology with the probable viral immuno-pathogenesis mechanism of SARS CoV-2. It also outlines the correlation between comorbidities and neurological complications, and lastly, it mentions the prophylaxis and vaccines used for treating COVID-19 patients.
KEYWORDS: Coronavirus; Comorbidities; COVID-19; Neurological complications; SARS CoV-2
| Copy the following to cite this article: Biswas J. An All-Inclusive Overview of COVID-19 (SARS-COV-2): Emphasising Immuno-Pathogenesis, Correlation with Comorbidities, Neurological Consequences, and Therapeutic Objectives. Biotech Res Asia 2025;22(3). |
| Copy the following to cite this URL: Biswas J. An All-Inclusive Overview of COVID-19 (SARS-COV-2): Emphasising Immuno-Pathogenesis, Correlation with Comorbidities, Neurological Consequences, and Therapeutic Objectives. Biotech Res Asia 2025;22(3). Available from: https://bit.ly/3KbRs9f |
Contextual Background
Coronaviruses (CoVs) are associated with the extended family of positive, encased, extensively diverse, and single-stranded RNA viruses.1,2 Coronaviruses were first perceived in the mid-1930s, and the first human coronavirus was notified in 1960, causing common cold-like symptoms.3–5 In 2003, a beta (β) – coronavirus emerged from bats and disseminated through the palm civet (mediator host). It was transmitted to humans in China’s Guangdong territory, where it was designated as the SARS (Severe Acute Respiratory Syndrome) virus, affecting approximately 8422 people, 916 of whom died. 4,6 In the middle of 2012, the initial instance of MERS (Middle East Respiratory Syndrome) was identified in Jeddah, Saudi Arabia. It emerged from bats, but the mediator host was a camel, and it affected 2994 individuals, 858 of whom died.4,7 Wuhan, China was the initial location to identify instances of the COVID-19 obtained from patients with pneumonia. Later, it spread globally to almost every country, creating a nationwide lockdown.4,8,9
The WHO (World Health Organization) designated the outbreak as COVID-19 (Novel Coronavirus Disease 2019), which is caused by the SARS CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2), on February 11, 2020. The assumed method of spreading COVID-19 is from one person to another through droplets expelled by infected individuals when they cough or sneeze nearby.1,10,11 The primary signs of COVID-19 include are pyrexia (fever), tussis (cough), emesis (vomiting), and diarrhea.1,12 The major health issues caused by SARS CoV-2 include and multiple organ failure, sepsis, ARDS (acute respiratory distress syndrome), septic shock, which are associated with co-morbidities (cancer, immunodeficiency, immunosuppressive disorders, cardiovascular diseases, diabetes, and asthma) (Table 1).13–16 The geriatric section and patients with co-morbidities or chronic diseases are specifically vulnerable populations.1,17 Nearly five years have passed since the first COVID-19 cases were reported in Wuhan, China. Over the past few challenging years, we’ve explored a variety of treatment approaches, implemented preventative measures, embraced social distancing, and fueled groundbreaking vaccine research. Unfortunately, in spite of these initiatives, especially concerning pharmacotherapy and patient management, over 775 million people worldwide have contracted SARS-CoV-2, and more than 7 million of those cases have resulted in death from COVID-19 infection. Furthermore, based on epidemiological mortality rate assessment research, the death rate has surpassed 18.2 million so far, and this deadly epidemic will claim many lives.18
Table 1: Symptoms, Disease Pathogenesis and Clinical Manifestations Observed in Different Stages of COVID-19.
| Stage / severity | Symptoms | Disease Pathogenesis | Hospitalization Required or not | ||
| Asymptomatic /Pre-symptomatic | SARS-CoV-2 test resulted in a positive outcome, yet there are no symptoms present. 19,20 | Viral replication 19,20 | Hospitalization is not required 19,20 | ||
| Mild Illness | Mild signs (include fever, cough, changes in taste or smell, or their complete loss); sore throat; general discomfort; headache; muscle pain; nausea; vomiting; and diarrhea. 19,20 | ||||
| No SOB (shortness of breath) or imaging findings. 19,20 | |||||
| Moderate Illness | Clinical assessment and imaging results indicate the presence of a lower respiratory tract infection. 19,20 | Viral replication 19,20 | Inflammation 19,20 | ||
| SOB (shortness of breath) or Affirmative imaging results. 19,20 | |||||
| O2 saturation ≥ 94%. 19,20 | |||||
| Severe Illness | Lower respiratory tract disease. 19,20 | Viral replication 19,20 | Inflammation 19,20 | Hypercoagulability 19,20 | Hospitalization required 19,20 |
| Lung infiltrates > 50%. 19,20 | |||||
| O2 saturation < 94%. 19,20 | |||||
| Respiratory rate 30/min. 19,20 | |||||
| Critical Illness | Multiple organ dysfunction/failure; septic shock; respiratory failure. 19,20 | Inflammation 19,20 | Hypercoagulability 19,20 | ||
| Intubated or ICU (intensive care unit) admission. 19,20 | |||||
Materials and Methods
A comprehensive review was conducted to evaluate the progression of the SARS-CoV-2 virus (COVID-19), its impact on public health, associated neurological complications, and the available treatments and vaccination approaches. The review was based on an extensive search of peer-reviewed literature published on the COVID-19. The following methods were employed:
Literature Search Strategy
A systematic search was performed across multiple electronic databases including PubMed, ResearchGate, Scopus, Semantic Scholar, and Google Scholar. The search strategy incorporated a broad set of keywords related to COVID-19, such as: “COVID-19”; “SARS-COV-2”; “Coronavirus”; “SARS-COV-2 Variants”; “SARS-COV-2 Taxonomy”; “COVID-19 Pathology”; “COVID-19 Diagnosis”; “COVID-19 Comorbidities”; “COVID-19 Cardio-Vascular Disorder”; “COVID-19 Hypertension”; “COVID-19 Diabetes”; “COVID-19 Neurological Disorders”; “COVID-19 Encephalopathy”; “COVID-19 Guillain-Barré Syndrome”; “COVID-19 Stroke”; “COVID-19 Treatments”; “COVID-19 Immunomodulatory Treatment”; “COVID-19 Antiviral Drugs”; and “COVID-19 Vaccines”. Studies were selected based on relevance, quality, and inclusion of information on the virology, pathology, comorbidities, neurological impact, and vaccine development significantly centred on the topic “COVID-19”.
Selection Criteria
This review article included original research articles, reviews and studies that provided information on the structure and taxonomy, as well as the immuno-pathophysiology of SARS-CoV-2. Additionally, it incorporated studies exploring the relationship between comorbidities and COVID-19, investigations into the neurological effects of COVID-19, and articles highlighting COVID-19 management as well as examining the development, efficacy, and safety of COVID-19 vaccines.
Results
Eligible studies, including full-text reviews and original research articles focused specifically on COVID-19, were critically reviewed to extract data on viral taxonomy, structural virology, immuno-pathogenesis, neurological complications, treatments, and vaccine development. The extracted data was then categorized into the following areas:
Taxonomy and Structural Virology
Information on the classification of SARS-CoV-2 within the coronavirus family and its structural composition and units.
Immuno-Pathophysiology of SARS-CoV-2
Insights into the mechanisms of SARS-CoV-2 viral entry and developing a disease severity.
Neurological Complications
Information on the incidence and types of neurological complications associated with COVID-19
Rapid Detection of COVID-19
Methods and technologies for the swift identification of COVID-19, including rRT-PCR tests, lateral flow immunoassay, and emerging diagnostic tools for early detection.
Impact of Comorbidities on COVID-19 Severity
Insights into how underlying conditions like diabetes, hypertension, and cardiovascular disease exacerbate the severity of COVID-19.
Treatment Strategies
An overview of antiviral agents, immunomodulatory treatments, neutralizing antibodies, convalescent plasma therapy, and antithrombotic therapy, along with their application in managing mild to severe COVID-19 cases.
Comprehensive List of COVID-19 Vaccines
An overview of available COVID-19 vaccines, highlighting their adverse effects, effectiveness, and efficacy against various SARS-CoV-2 variants.
By following this systematic approach, this review article offers a comprehensive summary of viral taxonomy, structural virology, immuno-pathogenesis, neurological complications, treatment strategies, and vaccine development , all focused specifically on COVID-19.
Discussion
Taxonomy, Structural Virology and Circulating Sars-Cov-2 Variants
The International Committee on Taxonomy of Viruses (ICTV) classified coronaviruses under the order Nidovirales and the family Coronaviridae and subfamily Orthocoronavirinae (Figure 1A).21 The ‘Coronavirus’ is named since the outer surface of the virus contains crown-like spike projections. 2 Based on serological evidence, the subfamily Orthocoronavirinae is divided into four genera: α-CoVs (alpha-coronavirus), β-CoVs (beta-coronavirus), ϒ-CoVs (gamma-coronavirus), and δ-CoVs (delta-coronavirus) (Figure 1A).4,22,23 Coronaviruses are generally nurtured in mammals (bats, camels, cattle, cats, etc.) and birds. Alpha and beta-coronaviruses infect mostly mammals, whereas gamma-coronaviruses primarily infect birds and a few mammals. However, delta-coronaviruses infect both. Animal coronaviruses largely infect domestic birds and animals, affecting productivity along with economic loss. 22,23 These animal coronaviruses include Porcine Epidemic Diarrhoea Virus (PEDV), Avian Infectious Bronchitis Virus (IBV), Transmissible Gastroenteritis Virus (TGEV), and Swine Acute Diarrhoea Syndrome Coronavirus (SADS‐CoV). Coronaviruses are unusually potent human infectors and could spread through from one person to another. The initial coronaviruses identified were IBV, which caused respiratory illness in chickens, and Human Coronavirus 229E and OC43 (HCoV OC43 and HCoV 229E), which caused common cold-like symptoms in humans. Several other human coronaviruses were identified, such as in 2002 [Severe Acute Respiratory Syndrome Coronavirus (SARS‐CoV)], in 2004 [Human Coronavirus (HCoV)-NL63], in 2005 [Human Coronavirus (HCoV)- HKU1], in 2012 [Middle East Respiratory Syndrome-Coronavirus (MERS‐CoV)], and the recent global pandemic [Severe Acute Respiratory Syndrome Coronavirus 2 (SARS CoV-2)]. 9,22,24–27
Coronaviruses are observed under an electron microscope as minute, spherical, enveloped particles 60 to 140 nm in diameter, made up of single-stranded RNA (26 to 32 kbs in length). 2,4,28 The coronavirus mRNA encodes the four structural proteins: Spike (S) protein, Nucleocapsid (N) protein, Membrane (M) protein, and Envelope (E) protein (Figure 1B). The spike glycoprotein (S-protein) projections are a vital characteristic feature of coronaviruses. 4 It forms the bulky glycosylated peplomers embedded in the lipid bilayer, mediates attachment to the Angiotensin-Converting Enzyme 2 (ACE2) receptor, and facilitates membrane fusion to initiate viral pathogenicity in the host cell. The embedded hemagglutinin-esterase (HE) dimer designs the minute spikes on the viral outer surface. The positive-sense viral RNA is integrated with the viral nucleotide N-protein to form the nucleocapsid, which plays an important role in viral replication and transcription. The hydrophobic trans-membrane protein (M-protein) is the most prominent viral surface glycoprotein and is assumed to be the central organiser for assembling the coronaviruses. The trans-membrane envelope glycoprotein (E-protein) is the minor component of the coronaviruses and plays a prominent role in virus assembly, host cell membrane permeability, and also virus-host cell interaction.2,4,22,28,29
Eventually, researchers discovered a worldwide dominant variant, D614G, which was associated with increased transmissibility but not the potential to cause severe illness.30 Several SARS-CoV-2 variations have been reported since then, some of which are regarded as variants of concern (VOCs) because they may result in increased transmissibility or pathogenicity. A categorization method for differentiating the new SARS-CoV-2 variations into variants of interest (VOIs) and variants of concern (VOCs) has been separately developed by the World Health Organisation (WHO) and the United States Centres for Disease Control and Prevention (CDC) (Table 2). 31 Fortunately, no SARSCoV-2 variant that satisfies the VOC requirements is currently in circulation. Certain Omicron lineages, such as EG.5, XBB.1.5, and XBB.1.16, are considered to be VOIs that are currently in circulation (Table 2).18 A number of Omicron lineages have been identified as presently circulating variants under monitoring (VUMs), such as XBB, XBB.1.9.1, and XBB.2.3. Information about the immunological escape, growth rate, and transmissibility of these new Omicron lineages is limited. These lineages have been associated with the following primary spike mutations: I332V, D339H, R403K, V445H, G446S, K444T, L452R, N450D, L452W, E180V, T478R, F486P, N481K, 483del, and E484K. More research and observation are necessary because the epidemiological and phenotypic effects of these mutations and associated lineages are still uncertain.18
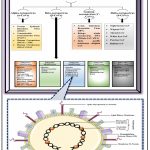 |
Figure 1: Illustrating the taxonomical and schematic structure of SARS CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2). |
Schematically representing the taxonomical classification of coronaviruses. Constructed using the following references: 2,4,9,21–27,32
Schematic structure of SARS CoV-2. Constructed using the following references: 2,4,22,28,29 and this figure was partially made using Servier Medical Art 33
Table 2: System of Classification for Differentiating the Emerging SARS-CoV-2 Variants.
| Variant Type | Variant Name | Lineage | First Detected | Spike Mutations | Important Points |
| Variants of Interest (VOIs) | XBB.1.5 | Omicron | The USA, or United States of America 18,34–36 | N460K,S486P, and F490S 18,34–36 | · Identical to the baseline in terms of transmissibility, immunity, and disease severity. 18,34–36· Poses no additional harm to public health and has a shallow risk of illness intensity and proliferation rate along with an average probability of antibody evasion. 18,34–36· Decreased the neutralising power of the currently accessible mRNA vaccines. 18,34–36 |
| XBB.1.16 | _ | _ | · The genetic makeup is comparable to that of the XBB.1.5 lineage. 18,34–36· No additional harm to public health. 18,34–36· Slow growth rate and a moderate chance of antibody evasion. 18,34–36· Has a shallow risk of illness intensity. 18,34–36 | ||
| EG.5 | _ | F456L, N460K, S486P, and F490S 18,34–36 | · The most common variant of SARS-CoV-2 globally. 18,34–36· Similarity in transmissibility to the initial sub-lineages of Omicron. 18,34–36· A low pace of growth and a moderate chance of antibody evasion. 18,34–36· Greater potential for antibody evasion, growth pace, and prevalence than others. 18,34–36 | ||
| BA.2.86 | Israel along with Denmark 18,34–36 | Hasenormous mutations in the spike protein 18,34–36 | · Propensity to cause a sharp rise in COVID-19 instances, but there would be no increase in the disease’s severity. 18,34–36· XBB-infected individuals’ convalescent plasma had sufficient neutralising activity against BA.2.86, indicating that XBB.1.5 monovalent COVID-19 vaccines could be effective against the BA.2.86 lineage. 18,34–36 | ||
| JN.1 | Sub-lineage of BA.2.86 | First detected on August 2023 18,34–36 | _ | · The most common sub-lineage of SARS-CoV-2 worldwide. 18,34–36· Minimal risk to the public’s health and no shifts in the severity of diseases or hospitalisation rates. 18,34–36· Despite instances of immunological escape, the monovalent XBB.1.5 booster immunisation is still advantageous. 18,34–36 | |
| Variants Under Monitoring (VUMs) | XBB, XBB.1.9.1, and XBB.2.3 | Omicron | _ | E180V, G446S, T478R, I332V, F486P, K444T, 483del, D339H, N450D, R403K, L452R, V445H, N481K, L452W, and E484K 18,34–36 | · Information about the immunological escape, growth rate, and transmissibility is limited. 18,34–36 |
| De-escalated SARS-CoV-2 variants | B.1.1.7 | Alpha | In the United Kingdom in September 2020 18,34–36 | N501Y, Y144P681H, and H69/V70 18,34–36 | · Demonstrated a 30% rise in viral contagiousness and transmissibility. 18,34–36· Capable of causing a serious COVID-19 infection. 18,34–36· Compared to SARS-CoV-2 wild type, the efficacy of vaccinations and monoclonal antibodies against this variation was decreased because of these mutations. 18,34–36· A ten-fold increase in ACE2 binding potential. 18,34–36 |
| B.1.351 | Beta | In South Africa in October 2020 18,34–36 | K417N,L18F, N501Y, E484K, D80A, A701V, and D215G 18,34–36 | · Demonstrated a 50% rise in viral contagiousness and transmissibility. 18,34–36· Capable of causing a severe COVID-19 infection. 18,34–36· Comparing these alterations to the SARS-CoV-2 wild type, vaccinations and monoclonal antibodies were less effective against this variation. 18,34–36· A two-fold increase in ACE2 binding potential. 18,34–36 | |
| P.1 | Gamma | In Brazil and Japan in November 2020 18,34–36 | K417T, E484K, AND N501Y 18,34–36 | · Accompanied by 30–40% rise in viral contagiousness and transmissibility. 18,34–36· Causing a serious case of COVID-19. 18,34–36· When compared to the wild type of SARS-CoV-2, these alterations reduced the effectiveness of monoclonal antibodies and vaccines against this variant. 18,34–36· A five-fold increase in ACE2 binding potential. 18,34–36 | |
| B.1.617 | Delta | In India, in December 2020 18,34–36 | L452R, R158G,P681R, T478K, D950N, and D614G 18,34–36 | · Viral infectivity and transmissibility increased by more than 90%. 18,34–36· Caused a serious COVID-19 illness. 18,34–36· While comparing the infected individuals to the preceding variations, the viral RNA load was significantly higher. 18,34–36· These mutations decreased the potency of the vaccination, yet it was still 80% effective against hospitalisation and serious COVID-19 illness. 18,34–36
· Hospitalisation and mortality rates from the Delta variant disease were found to be reduced when monoclonal antibodies, such as imdevimab, sorovimab, and casirivimab, were administered. 18,34–36 · A two-fold increase in ACE2 binding potential. 18,34–36 |
Immuno-Pathophysiology of SARS-COV-2
The genome of a coronavirus consists of about 30,000 nucleotides, and its structural proteins are encoded by its mRNA.1,28 Earlier research shows that the main targets of SARS-CoV are the host’s pulmonary system, especially the macrophages, alveolar epithelial cells, vascular endothelial cells, and airway epithelial cells, which have Angiotensin-Converting Enzyme 2 (ACE2) receptors. In a similar manner, SARS-CoV-2 utilizes the ACE2 receptor on the host cell to facilitate entry (Figure 2). When the virus attaches to the ACE2 receptor, it reduces its expression and causes the lung cells to down-regulate, which results in acute lung damage.37–46
The two subunits that make up the spike (S) glycoprotein are S1 and S2. An ATD (Amino-Terminal Domain) and a RBD (Receptor-Binding Domain) make up the subunit S1. 43,47–49 The RBD starts the viral infection phase by attaching itself to the host cellular target ACE2. RBD connects to the cellular receptor ACE2, which triggers the endocytosis of SARS CoV-2 virion and exposes it to the host endosomal proteases furin and trypsin. HR1 and HR2, the Heptad Repeat Regions, and the FP (Fusion Peptide) region make up subunit S2. After the subunit S1 is broken down within the endosome, FP emerges and enters the host membrane. The viral payload is subsequently released into the host cell’s cytoplasm when the subunit S2 coincides on itself to connect the two heptad repeat sections in proximity, triggering the membrane fusion (Figure 2). 43,50–52 Biophysical tests and computer modelling show that the RBD of SARS CoV-2 attaches to ACE2 with greater proximity than that of SARS-CoV. Like MERS-CoV and HC-OC43, the spike glycoprotein of SARS-CoV-2 forms a furin-like cleavage site, increasing the pathogenicity in comparison to SARS-CoV. TMPRSS2 (Transmembrane Protease Serine 2) is necessary for the recognition of the SARS CoV-2 spike glycoprotein and for facilitating the viral entrance into the host cell. 43,53,54 After the virus enters the host cell’s cytoplasm by endocytosis, the SARS CoV-2 RNA gets released and processed into two polyproteins and structural proteins that eventually replicate the virus’s genome. 1,28 The replicating viral component mediates viral genome replication and is composed of an exonuclease N, a helicase, an RdRp (RNA-dependent RNA polymerase), and other related proteins.55 The mechanism by which N protein encapsidates duplicated genomes in the cytoplasm results in nucleocapsids, which then consolidate within the membrane to self-assemble into new virions. The ER (Endoplasmic Reticulum) and Golgi Apparatus membranes both include the recently synthesised structural glycoproteins in the cytoplasm, which are then transferred to the ERGIC (Endoplasmic Reticulum-Golgi Intermediate Compartment). 1,28,56 Finally, the novel virions are fused with the plasma membrane via exocytosis, and the virus is released (Figure 2).1,28
The released virus instigates the host cell to initiate pyroptosis and release ATP, ASC oligomers, Interleukin-1 (IL-1), and nucleic acids, which constitute the Damage-Associated Molecular Patterns (DAMPs). Adjacent epithelial cells, alveolar macrophages, and endothelial cells recognise the released danger signals (DAMPs), which trigger pro-inflammatory cytokines (such as TNF [Tumor Necrosis Factor-alpha]–α; TGF [Transforming Growth Factor-beta]–β; IFN [Interferon-alpha]–α,γ; Interleukin [IL]–1β, 6, 12, 18, 33, 281) and chemokines (such as CCL [C‑C motif chemokine ligand]–2, 3, 5, and CXCL [C-X-C motif chemokine]–8, 9, 10).1,13,43,57–59 T cells, macrophages, and monocytes are drawn to the infection site by pro-inflammatory chemokines and cytokines, which also promote further inflammation through the production of interferon-gamma (IFN-γ) by T cells and a pro-inflammatory feedback looping mechanism. When the spike glycoprotein of SARS CoV-2 binds to the ACE2 receptor, it affects the RAS and causes vascular permeability. This can result in a faulty immunological response where immune system cells build up in the lungs, producing excessive pro-inflammatory cytokines and adversely impacting the lung (Figure 2). 1,28,37–39,43,56 The subsequent cytokine storm spreads to neighboring organs, leading to malfunction or failure of several organs. Moreover, through Antibody-Dependent Enhancement (ADE), non-neutralizing antibodies produced by B lymphocyte cells (B cells) increase the SARS CoV-2 disease and exacerbate organ dysfunction or injury. In favorable immune responses, virus-specific CD4+ and CD8+ T cells draw in and destroy the virus at the onset of inflammation, preventing it from spreading. The alveolar macrophages recognizes the viral cells and neutralized them. They phagocytose the apoptotic cells to eliminate the virus and maintain minimal lung damage with resultant recovery.1,13,43,57–59
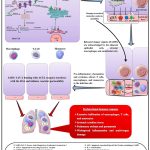 |
Figure 2: The plausible immunopathogenesis mechanism of SARS-CoV-2. |
Constructed using the following references:1,13,28,37–40,42–54,56–60 and this figure was partially made using Servier Medical Art33
Rapid Detection of COVID-19
Rapid diagnosis or detection is a predominant health care function since it imparts details about the health issues of a patient and provides consecutive decisions.61 The vital advantages of rapid diagnostic techniques are the prospect of rapid intervention and a focused solution to potential problems. Similarly, COVID-19 can be detected using rapid diagnostic techniques like RT-PCR (real-time polymerase chain reaction), primary screening through an immunodiagnostic test (rapid antibody test), and finally by Artificial Intelligence (AI)-based image processing techniques such as chest X-ray and CT (Computed Tomography) scan (Figure 3A).4,61,62
The most recent technique for qualitatively and quantitatively identifying the SARS-CoV-2 nucleic acid in both upper and lower respiratory swab samples in the intense period of illness is real-time reverse transcription-polymerase chain reaction, or rRT-PCR.4,61,62 One of the best and most efficient scientific techniques for tracking, treating, and analyzing the COVID-19 is rRT-PCR. By using particular primers for the COVID-19 viral genes, cDNA is produced using this technique from the COVID-19 virus’s retrieved RNA. Specific areas of cDNA (target genes) are amplified and identified using various fluorescent dyes (Figure 3B). The use of rRT-PCR assays has several important advantages, but the main one is that the amplification and analysis would take place in a contained system, reducing the likelihood of false-positive findings.63–66
After contracting SARS-CoV-2, the patient produces IgM or IgG antibodies which are specific for the viral antigens to the receptor binding domain (RBD), the spike glycoprotein (S1, S2 subunits), or the nucleocapsid (N) protein.67,68 IgM first appears after infection and remains detectable for a couple of days and then IgG follows. All current quick SARS-CoV-2 antibody assessments rely on the ability of the N, S1, S2, or RBD domain of the SARS-CoV-2 spike protein to associate with IgM or IgG antibodies in the patient’s blood.68–70 Time-dependent detection of IgM or IgG isotypes only reaches high sensitivity around three weeks after symptoms start.68,71 Lateral flow detection [Lateral Flow Immunoassay (LFIA)] is used in rapid SARS-CoV-2 antibody testing (Figure 3C).68,72 The blood sample taken from the person who has a suspected COVID-19 infection is used for LFIA. In lateral flow testing, an absorbent pad attached to the leading edge of the strip allows an antigen or antigen-antibody complex to flow across it. The polymeric strip’s different zones are travelled by a liquid sample that contains the analyte. As a sponge, the sample pad allows fluid to go to the next conjugate pad after it has been moistened. A chemical reaction between an antigen and an antibody can be carried out using the chemicals included on the conjugate pad. The antigen passes through the pad, leaving a mark behind. The antigen continues to flow via the test and control lines. Following its passage through these lines, the fluid enters a porous material that serves as a disposal storage.72 Observations are made based on the state of the patient and can be classified into the following categories: negative (just the control sign formed), IgM positive (both the IgM mark and control markings formed), IgG positive (both the IgG mark and control markings formed), or just both IgG and IgM positive (both the IgM and IgG markings developed along with the control line) (Figure 3C).72,73
 |
Figure 3: Quick methods for identifying or diagnosing COVID-19. |
Rapid detection or diagnostic methods for detecting COVID-19. Constructed using the following references: 4,61,62,74 and this figure was partially made using Servier Medical Art33
The classic rRT-PCR (real-time reverse transcription-polymerase chain reaction) procedure is demonstrated, in which viral RNA is isolated and transformed into cDNA. By using rRT-PCR, specific regions of cDNA (target genes) are amplified and identified. Constructed using the following references: 63–66 and this figure was partially made using Servier Medical Art33
Steps representing LFIA (lateral flow immunoassay)-based COVID-19 diagnosis. Constructed using the following references:67–72,74 and this figure was partially made using Servier Medical Art33
Comorbidities of Cardiovascular Disease, Diabetes, and Hypertension in the Context of COVID-19
Patients with COVID-19 are more likely to have acute cases and greater fatality rates if they have underlying comorbidities and are older than 70. The prominent comorbidities that are associated with COVID-19 are cardiovascular diseases, diabetes mellitus, and hypertension.16,75–78 This can be suggested by a study conducted by Li et al.,17 among 1527 COVID-19 patients admitted; 17.1% had hypertension, 16.4% had cardiovascular disease, and 9.7% had diabetes mellitus. 17 The survey by Huang et al.,13 comprised 41 hospitalized patients with a confirmed COVID-19 infection; 32% of these patients had concomitant conditions such as cardiovascular illnesses (15%), diabetes (20%), and hypertension (15%).13 Similarly, the study of Wang et al.,79 suggests the prevalence of one or more comorbidities (likely 31% of patients with hypertension, 14.5% with cardiovascular diseases, and diabetes 10.0%) in 64 individuals from 138 hospitalized COVID-19 patients.79 In general, COVID-19 infects both sexes; however, men have a 3.6% case fatality rate compared to women’s 1.6%.80 Patients with cardiovascular diseases who contracted COVID-19 were three times more likely to experience an acute illness or require admission to the intensive care unit (ICU) than those with diabetes mellitus or hypertension.17,76,80 According to a Chinese Centre for Disease Control and Prevention81 study, 44,672 patients out of 72,314 distinct records had COVID-19 confirmed in them; 1,023 of these patients died, resulting in a crude case fatality rate of 2.3% (number of confirmed mortality divided by the overall number of confirmed infections). Additionally, a greater fatality frequency was noted for individuals with underlying comorbidities: 10.5% of patients having cardiovascular disorders, 7.3% of patients with diabetes, and 6.0% of patients with hypertension.81
A survey by Chen et al.,82 involving 99 patients confirmed with COVID-19 revealed that 51% of the admitted patients were detected with chronic medical illness (40% with cerebrovascular or cardiovascular disease). 82 A retrospective multi-centre study by Ruan et al.,83 included 150 patients with confirmed COVID-19 and found that people with cardiovascular diseases have a much higher risk of dying from SARS-CoV-2 infection.83 Secondary infections were seen in 1% (1/82) and 16% (11/68) of the patients in the discharge group and death groups, respectively. According to laboratory results, white blood cell counts, platelets, absolute values of lymphocytes, total bilirubin, albumin, blood creatinine, blood urea nitrogen, cardiac troponin, CRP (C-reactive protein), IL-6 (interleukin-6), myoglobin, and all were significantly different between the two groups.83 According to reports, SARS-CoV-2 is thought to infect the myocardium and cause myocarditis because of the myocardial infiltration by the interstitial mononuclear inflammatory cells that were found there during the post-mortem biopsy.45,80 In some instances, acute myocarditis with reduced systolic function is observed post-COVID-19. 80,84,85 Research on cardiac biomarkers suggested that hospitalized COVID-19 patients had a significant probability of cardiac injury. 45,80,86,87 Myocardial injury is probably accompanied by myocarditis and/or ischemia and is a vital predictive aspect of COVID-19.80 A single-centre observational study by Shi et al.,87 revealed the significance of cardiac injury in 416 admitted COVID-19 patients, of whom 82 (19.7%) died of cardiac injury. Admitted older patients with more underlying comorbidities and high levels of leukocytes are reported to have cardiac injury due to greater hs-cTnI (high sensitive cardiac troponin I).87 A retrospective single-centre case series by Guo et al., 86 where 187 patients with confirmed COVID-19 were admitted to the hospital; 66 (35.3%) of these patients had preexisting cardiovascular conditions (cardiomyopathy, hypertension, and coronary heart disease), and 52 (27.8%) had myocardial damage, which suggests elevated cTnT (cardiac troponin T) readings.86
Diabetes is one of the vital co-factors of morbidity and fatality throughout the world and is accompanied by various microvascular and macrovascular problems that eventually affect the overall patient’s survival. 76 The prevalence of diabetes was found to be 35% (mean age, 79.5 years) in an analysis of a randomly chosen sample of fatal SARS-CoV-2 patients in Italy. 88,89 A comprehensive retrospective study of 1591 SARS-CoV-2 patients admitted in ICUs in Lombardy, Italy, over the course of four weeks revealed an occurrence of T2DM (Type 2 Diabetes Mellitus) of 17%. 88,90 In T2DM, apart from the pro-inflammatory storm of cytokines, a disproportion between coagulation and fibrinolysis occurs, with an inflated proportion of clotting factors and relative fibrinolytic system inhibition.76 Both T2DM and insulin resistance are accompanied by endothelial malfunction and inflated platelet accumulation and activation, which instigate the progression of a hypercoagulable pro-thrombotic state.76,91 Older people with COVID-19 are noted to have impaired T-cell and B-cell functions and an inflammatory cytokine storm. Consequently, T2DM by itself or in conjunction with advanced age and other comorbidities may encourage unchecked SARS CoV-2 proliferation, increasing the disease’s lethality.76,92 Since the elderly population is most impacted by COVID-19 and hypertension is particularly widespread in older people, the prevalence of hypertension in these patients is not wholly uncommon.77,93 Recent studies indicate that a high death rate was detected in COVID-19 individuals who had underlying comorbidities, most notably diabetes, hypertension, and cardiovascular disorders.76,77,93,94
COVID-19 and Pathophysiological Aspects of Neurological Complications
Neurological problems are prevalent in COVID-19 patients, especially in hospitalized patients, who exhibit greater rates than in patients with less severe disease. 95,96 Smell and taste problems, intracranial haemorrhage, ischemic stroke, encephalopathy, encephalomyelitis, and neuromuscular illnesses are among the most frequent neurological consequences.97,98 A variety of factors, such as SARS-CoV-2 neurotropism, endothelial dysfunction, hypercoagulability, hypoxia, systemic illness, and response, may cause the development of neurological manifestation signs.97
Neurological problems can result from the SARS-CoV-2 virus’s ability to enter the central neuronal system and affect both neurons and glial cells. Various patho-mechanisms give rise to neurological diseases. Findings indicate that the initial mechanism by which SARS-CoV-2 enters host cells is the ACE2 (angiotensin-converting enzyme type 2), which is found on the cell periphery of many tissues. 97,99–104 By adhering to ACE2 in a variety of organs, including as the neurological system, skeletal muscle, and vascular endothelium, SARS-CoV-2 can enter the central neuronal system and impair blood vessels. 97,103,104 Massive intracerebral haemorrhage can emerge from ruptured blood vessels triggered by disruption to the cerebral endothelium and a rise in cerebral blood pressure. 97,103 However, concomitant COVID-19 infection, hypercoagulability, and thromboembolic circumstances may result in an ischemic stroke. 97,105 The brainstem—which comprises the paraventricular nuclei and the nucleus of the solitary tract—is where ACE2 is mostly expressed in the brain and is responsible for cardiac and vascular function. 97,101,102 Additionally, it has been demonstrated that an infection can spread via synaptic connections from peripheral neuronal to the central neuronal system. 106,107 The dissemination of SARS-CoV-2 through the olfactory nerve may be an example of how disease proceeds along a neural route because of the peculiar structure of the olfactory nerve including associated olfactory fibres inside the nasal canal. Through the olfactory nerve and bulb routes, coronaviruses can colonize the nasal passages before entering the brain and cerebrospinal fluid, resulting in an inflammatory and demyelinating reaction. 100,101 Another source of negative consequences is the development of an extremely wide systemic inflammatory response (SIRS), which results in an excess of interleukin (IL-6,12,15) and TNF-α (tumour necrosis factor-alpha). This inflammation triggers glial cell activation and a strong proinflammatory phase in the central nervous system, which in turn causes severe hypoxia, leading to cerebral oedema, cerebral vasodilation, and ischemia.97,106,107
The most prevalent neurological side effects are problems related to taste and smell. Anosmia and taste abnormalities were identified as preliminary markers of COVID-19 in one study, affecting more than 80% of patients.108 Smell dysfunction impacted 48% of patients, according to a meta-analysis of 83 research involving over 27,000 participants (95% CI 41.2–54.5).109 Anosmia is reported by younger patients more frequently than by older ones. Additionally, women experience it more frequently than men do. 110 The majority of patients have either significant improvement or overall regression in 2-3 weeks, indicating a typically good prognosis for smell and taste impairments linked to COVID-19. As a result, the prognosis is probably more favorable than it would be for other aetiologies of smell disorders. However, in 10% to 20% of cases, severe and persistent deficiencies persist.98 Fortunately, most COVID-19 patients experience a natural recovery of their taste and smell problems, indicating that no special management is needed. However, smell training approaches could be helpful as a treatment if smell abnormalities last longer than four weeks.97,111
In most cases, patients in critical condition acquire encephalopathy. Delirium associated with encephalopathy is extremely uncommon and may manifest early or even without symptoms. In a cohort investigation involving 2088 COVID-19 individuals admitted to intensive care units, 55% of patients experienced delirium.112 Shah et al.,113 found that out of 12,601 hospitalized patients, 1092 (8.7%) had acute encephalopathy.113 In an alternate study, 509 hospitalized COVID-19 patients were found to have encephalopathy in 31.8% of them.95 It usually has a multifaceted etiology. Older male patients who have previous instances such as neurological ailments, cancer, cerebral vasculitis, diabetes, renal diseases, dyslipidemia, cardiovascular disease, hypertension, or smoking are primarily affected. 95,97 Indications of encephalopathy and encephalitis include neuropsychological issues, agitation, delirium, motor impairments linked to extrapyramidal manifestations, abnormal coordination, seizures, diminished consciousness, and specific neurological impairments.97,98
In COVID-19, stroke appears to be slightly less prevalent.114–117 Among hospitalized patients, the proportions of intracranial haemorrhage were 0.2–0.9%, and the rates of ischemic stroke associated with COVID-19 were 0.4–2.7%.118,119 Additionally, there are reported cases of cerebral venous thrombosis (CVT) in COVID-19 individuals. 120,121 In a retrospective analysis involving more than 13,000 patients, twelve people with CVT were found within three months, indicating 8.8 cases per 10,000 patients. 122 A comprehensive evaluation of 34,331 hospitalized patients infected with SARS-CoV-2 estimated the prevalence of CVT to be 0.08% (95% CI 0.01–0.5).123 The majority of the time, a stroke occurs one to three weeks after COVID-19 symptoms initially manifest.122,124–126
In both the moderate and severe COVID-19 variations, myalgia is frequently experienced.97 It has been reported in 22–63% of patients,98 and in more extreme instances (19 vs. 5%), it is accompanied by elevated creatinine kinase (CK) levels; myopathy, or muscle injury, is also suspected.110 A rare and infrequent repercussion of COVID-19 is severe rhabdomyolysis.127 In 0.2% of patients, it was noticed, and in 13.7% of cases, elevated CK levels were found. Within five to seven days of the SARS-CoV-2 infection-induced fever, three Italian patients experienced prevalent myasthenia including positive acetylcholine receptor antibodies.128 While it is hypothesized that myasthenia may be caused by the immune system’s reaction to SARS-CoV-2, another possibility is that the infection just catalysis the onset of symptoms in individuals who already have myasthenia. Amyotrophic lateral sclerosis (ALS) cases have also been shown to involve an exacerbation of a pre-existing neuromuscular condition. It indicates that people with neuromuscular disorders do not have a significantly higher risk of contracting SARS-CoV-2 disease.98 Peripheral nerve abnormalities, inflammatory/autoimmune muscle disorders, and neuromuscular junction issues should all be managed according to the current guidelines. Immunoglobulin therapy and plasmapheresis are advised. It is recommended to postpone the use of rituximab or long-term oral immunosuppressive medicine in accordance with the patient’s clinical status and medical background. 97,98
About 0.4% of COVID-19 patients experience Guillain-Barré syndrome (GBS), which usually appears five to ten days after the viral infection.129 It implies that GBS is a parainfectious condition based on the appearance of the initial COVID-19 manifestations.130 It has been observed in specific studies that the symptoms are more intense and might manifest prematurely than those reported in conventional GBS. 131 In one study, mechanical ventilation was necessary for three out of five patients.131 In a span of one month, three hospitals in Italy’s northern region admitted almost 1200 individuals with COVID-19, but only five cases of GBS were found.129 Within one to four days, the majority of COVID-19 and GBS patients manifest progressive limb weakness.132 For GBS management in COVID-19, lacks particular recommendations. Patients should receive the same treatment as other individuals with GBS.97
Therapeutic Options for the Treatment of COVID-19
Since the pandemic outbreak, COVID-19 treatment has shifted rapidly, and it currently consists mainly of antiviral and immunomodulatory medications. Remdesivir and nirmatrelvir-ritonavir are two varieties of antivirals that have been reported to be most effective when used early in an illness (like outpatient treatment) and in cases of less acute illness. Janus kinase inhibitors Interleukin-6, and Dexamethasone, are examples of immunomodulatory therapies that work best when used in cases of extreme illness or critical condition. Due to the appearance of viral variations that are not expected to respond to these treatments, the significance of anti-SARS-CoV-2 monoclonal antibodies has decreased, and opinions regarding the use of convalescent plasma are still divided. The following sections cover the wide spectrum of available treatments, categorized by the classification of agent: antiviral medications, immunomodulators, neutralizing antibodies and convalescent plasma, and antithrombotic treatment. Patients undergoing various treatments have been explored with varying degrees of disease severity: mild to adequate COVID-19 in non-hospitalized patients; serious COVID-19 in hospitalized patients who need additional oxygen; and severe conditions in patients who need non-invasive or mechanical ventilation.19 The discussion mostly centres on randomized trials, which have contributed significantly to the current treatment paradigms. Every therapeutic section ends with a reference to the National Institutes of Health (NIH) COVID-19 Treatment Guidelines, which offer insight into the recommended usage of each therapy (Table 3).19,133
Primary Antiviral Agents utilized in the Management of COVID-19
Individuals with mild COVID-19 disease typically exhibit indications including myalgia, a high temperature, cough, and sore throat; on the other hand, patients with moderate disease show signs of lower respiratory tract involvement on radiographic or clinical examinations while still maintaining an oxygen saturation level of at least 94%. 134,135 The majority of these individuals can be safely treated in outpatient settings, such as emergency rooms, clinics, and telemedicine consultations.136,137 One of the first medications to show efficacy in a randomized controlled trial (RCT) during the early phases of the global outbreak was Remdesivir, a drug that inhibits viral RNA-dependent RNA polymerase. 19 The effectiveness of two oral anti-SARS-CoV-2 antiviral medications, molnupiravir and nirmatrelvir-ritonavir, provided to outpatients was assessed in two seminal phase 3 randomised trials, the EPIC-HR and MOVe-OUT trials. Molnupiravir and nirmatrelvir-ritonavir decreased hospitalization or mortality by 30 and 89%, respectively, when administered to unvaccinated people with COVID-19 disease (mild to adequate) within five days of apparition of symptoms and who were at risk of disease development.138,139 Hospitalization for symptomatic COVID-19 disease or non-COVID-related reasons may also be necessary for patients with mild-to- adequate illness. Compared to patients treated as outpatients, these patients had a higher chance of developing a severe clinical manifestation and a faster rate of disease development. 135 The clinical efficacy of molnupiravir and nirmatrelvir-ritonavir in patients hospitalized having mild-to-moderate COVID-19 disease was evaluate in three real-world studies conducted in Hong Kong and Japan. The majority of the patients involved in the research were older than sixty years, and their recommended immunization rates ranged from 10 to 80%.140–142 In comparison to controls that were matched, molnupiravir and nirmatrelvir-ritonavir were consistently linked to a 40–55% decreased risk of clinical deterioration. Additionally, it was found that nirmatrelvir-ritonavir and molnupiravir were linked to 66–90% and 52–69% decreased risk of death, respectively.140–142 An overview of the primary antiviral medications used to treat COVID-19 is discussed in (Table 3).
Primary Immunomodulatory Agents utilized in the Management of COVID-19
Severe and serious COVID-19 disease is treated with immunomodulatory substances, which include Janus kinase inhibitors (like baricitinib), interleukin-6 inhibitors (like tocilizumab), and corticosteroids.135,143 Corticosteroids were the first medications to improve survival in the treatment of COVID-19.144 It is advised to add interleukin-6 or Janus kinase inhibitors to corticosteroids in individuals whose condition progresses quickly and they show signs of systemic inflammation, such as increasing C-reaction protein. In summary, current research supports the application of remdesivir for the whole range of pulmonary assistance in hospitalized patients with serious and potentially fatal illnesses. Patients using low-flow supplemental oxygen should have their use of oral antivirals carefully assessed. In their clinical care, corticosteroids and other immunomodulatory therapies are indispensable.135 The primary immunomodulatory medications used to treat COVID-19 are summarized in (Table 3).
Neutralizing Antibodies and Convalescent Plasma Therapy
During the early stages of the global outbreak, antibody therapy proved to be a powerful treatment and prevention strategy for COVID-19. Convalescent plasma (CP) from recovered COVID-19 sufferers and monoclonal antibodies (mAbs) that aimed the S1 region of the SARS-CoV-2 spike protein were the two types of antibody products used. Pre-exposure prophylactic for immunocompromised individuals with the anti-SARS-CoV-2 monoclonal antibodies tixagevimab and cilgavimab (EvusheldTM) was authorized by the FDA. Many anti-SARS-CoV-2 treatments with monoclonal antibodies were approved, encompassing bamlanivimab plus etesevimab, bebtelovimab, sotrovimab, and casirivimab plus imdevimab. As the prevalence of various variants of concern (VOCs) increased, the pseudo-viral and in vitro viral neutralization activities of the monoclonal antibodies were utilized to forecast medical efficacy. In 2020, VOCs dominated the strains; however, numerous mAbs continued to have a neutralizing effect against B.1.1.7 (Alpha).19 But only bebtelovimab and sotrovimab retained relevance after Omicron (B.1.1.529) was fixed at the end of 2021, and not any of the approved mAbs sustained cross-neutralizing activity after Omicron subvariants continued to evolve in 2022.145 Consequently, the anti-SARS-CoV-2 monoclonal antibodies (mAbs) are not anymore used for pre-exposure prophylaxis, prophylaxis, or for the COVID-19 treatment.19,133
Antithrombotic Therapy
In the initial pandemic phase, hospitalized COVID-19 suffers experienced a higher prevalence of venous thromboembolism (VTE) episodes, which were most likely attributed to thromboinflammation. 146 It is difficult to establish the precise rate when compared to historical controls, although it has been estimated to be as high as three times the adult hospitalization baseline rate.147 The effectiveness of empiric anticoagulation and VTE prophylaxis has been assessed in numerous trials in addressing the elevated load of VTE (venous thromboembolism). The OVID and ETHIC randomized controlled trials (RCTs) examined individuals who were not hospitalized and contrasted enoxaparin or the conventional treatment. None of the two trials demonstrated effectiveness in lowering hospitalization or mortality, and both terminated prematurely.148,149 Unless there is a contraindication, prophylactic-dose heparin is advised for anticoagulation in hospitalized patients. Relying on the outcomes of randomized controlled trials assessing the application of anticoagulant therapy in non-critically condition and severely diseased hospitalized patients, the NIH (National Institutes of Health) COVID-19 Treatment Guidelines suggested therapeutic-dose heparin for adults who need traditional oxygen but not critical care, but only in cases where the D-dimer concentration is raised and there aren’t any circumstances which boost the likelihood of blood loss.. Moreover, prophylactic-dose heparin should be administered to individuals needing intensive care or high-flow oxygen rather than therapeutic-dose heparin (except when there are restrictions, such as confirmed thromboembolic disease).19,133,150,151
Table 3: An Overview of the Primary Antiviral Medications and Immunomodulatory Therapy Used to Treat COVID-19.
| Agent Type | Drug Name | Type (Delivery Route) | Eligible Patients | Information about the Drug | Significant Trials | An Overview of Data | NIH (National Institutes of Health) COVID-19 TreatmentGuidelines Suggestions |
| Antiviral Medications | Remdesivir | Small molecule (intravenous injection) 152 | Outpatientsb ≤ 7 days of symptom onset, or inpatients 152[b = Non-hospitalized individuals with mild-to-adequate COVID-19 are highly likely to escalate to critical COVID-19, potentially leading to hospitalization or even death.] | One of the first medications to show efficacy in a randomized controlled trial (RCT) during the early phases of the global outbreak was Remdesivir, a drug that inhibits viral RNA-dependent RNA polymerase. 19 | ACTT-1 153 | Included 1,062 hospitalized, unvaccinated patients who needed supplemental oxygen. 153 The results of the trial indicated that the remdesivir group recovered more quickly than the placebo group 153—10 days compared to 15 days—and that the group that needed traditional oxygen supplementation and those who started experiencing symptoms within 10 days of each other benefitted most from remdesivir. 153 | First-line treatment for hospitalized COVID-19 patients (in combination with immunomodulators if oxygen is required by the patient) and second-line treatment (subsequent nirmatrelvir-ritonavir) for vulnerable outpatients. 19,133,154 |
| CATCO 155 | In Canada, an open-label randomized controlled trial (RCT) revealed a lower risk of transition to mechanical ventilation but no decrease in death when compared to standard treatment. 155 | ||||||
| SOLIDARITY 156 | A multinational study consortium found that patients who were mechanically ventilated at enrolment did not have a lower death rate; however, patients who needed supplementary oxygen but were not mechanically ventilated did demonstrate a minor mortality advantage (14.6% versus 16.3%, р = 0.03). 156 | ||||||
| PINETREE 157 | Recruited unvaccinated outpatients were possessed at an elevated risk of advancement because of their age or comorbidities and who were within seven days of the onset of symptoms. The trial’s findings showed that, in comparison to a placebo, a three-day course of remdesivir considerably reduced hospitalization and mortality from all causes (0.7% vs 5.3%, р = 0.008). 157 | ||||||
| Nirmatrelvir-Ritonavir | Small molecule (oral) 152 | Outpatientsb ≤ 5 days of symptom onset 152[b = Non-hospitalized individuals with mild-to-adequate COVID-19 are highly likely to escalate to critical COVID-19, potentially leading to hospitalisation or even death.] | Ritonavir, a pharmacologic enhancer, is used in conjunction with nimatrelvir, a medication used orally which inhibits the protease of SARS-CoV-2. 19,138 | EPIC-HR 138 | Recruited high-risk, unvaccinated outpatients and, in comparison to a placebo, showed an 89% decrease in the likelihood of being hospitalized or mortality. 138 | For high-risk, outpatient patients, oral nirmatrelvir-ritonavir is recommended as the initial course of treatment. 19,133,154 | |
| EPIC-SR 158 | The study assessed the medication’s effectiveness in patients with standard risk (those who were at risk but had received vaccinations or did not have any factors that increased their chance of risk advancement). Hospitalization rates were low for patients in both arms of this trial, and there was no benefit of the medicine on long-term symptom relief. 158 | ||||||
| Retrospective Cohort Study 159 | Research conducted on highly-risk, vaccinated outpatients revealed that the nirmatrelvir-ritonavir group had a 45% proportional threat decrease in the cumulative outcome of a visit to the hospital emergency department, hospitalization, or death in comparison to the placebo category (7.87% versus 14.4%, р < 0.005), indicating its continued relevance for clinical practice. 159 | ||||||
| Molnupiravir | Small molecule (oral) 152 | Outpatientsb ≥18 years old and ≤ 5 days of symptom onset 152[b = Non-hospitalized individuals with mild-to-adequate COVID-19 are highly likely to escalate to critical COVID-19, even death.] | A prodrug of the minuscule molecule N-hydroxycytidine, which causes mutations to build up and depletes the viral viability of SARS-CoV-2. 19,139 | MOVe-OUT 139 | Assessed 1,433 vulnerable, unvaccinated outpatients. A 31% comparative risk decrease was observed in the 30-day hospitalization or mortality rate from all causes in the group receiving molnupiravir compared to the group receiving a placebo (7.3% versus 14.1%, р = 0.001). 139 | Molnupiravir is only recommended when remdesivir and nirmatrelvir-ritonavir are not capable of being administered. 19,133,154 | |
| PANORAMIC 160 | Molnupiravir did not reduce the rate of hospitalization or mortality among vulnerable outpatients, the majority of them had received vaccinations. This finding is less conclusive; due to the open-label nature of the trial, which may have influenced the research participants reported symptoms, the drug-treated group’s self-reported recovery time was significantly reduced.160 | ||||||
| Immunomodulatory Therapy
|
Corticosteroids | Small molecule (intravenous injection) 152 | Hospitalized patients needing non-invasive or mechanical ventilation, high-flow oxygen, or conventional oxygen. 19,152 | Corticosteroids were the first drugs to be shown to improve survivability during the management of COVID-19. 135 | RECOVERY 144 | An open-label study involving 6,425 hospitalized unvaccinated individuals compared standard treatment alone with a ten day regimen of dexamethasone. Results showed that those receiving supplemental oxygen (23.3% against 26.2%) and those necessitating mechanical ventilation (29.3% against 41.4%) had significantly lower 28-day death rates when compared to standard therapy in solitude. 144 There was no alteration seen for people who did not require additional oxygen, and in this group, dexamethasone was trending towards harm. 144 The normal amount of dexamethasone employed in the RECOVERY trial was 6 mg/day; however, there was considerable debate as to whether higher doses may be even more beneficial. But compared to the standard dosage of 20 mg of dexamethasone, those receiving a high dose had a higher death rate among patients needing conventional oxygen assistance. 161 Although large dosage of dexamethasone trials are presently being conducted in critically suffering patients, the data currently indicate that conventional dosage of dexamethasone is appropriate to be used in healthcare settings. 19,133 | For hospitalized patients needing non-invasive or mechanical ventilation, high-flow oxygen, or conventional oxygen, dexamethasone is still the fundamental component of COVID-19 therapy and is advised as first-line treatment. 19,133,154 |
| Interleukin-6 (IL-6) Inhibitors | Anti-IL-6R mAb (monoclonal antibody) [intravenous injection] 152 | Inpatients receiving systemic corticosteroids, requiring oxygen support, invasive or non-invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). 19,152 | The Food and Drug Administration (FDA) in the United States has formerly authorized monoclonal antibodies (mAbs) that block the proinflammatory cytokine interleukin-6 (IL-6) for the relief of autoimmune diseases such as cytokine release syndrome and rheumatoid arthritis associated with CAR-T (chimeric antigen receptor T cell) treatment. A recombinant, humanized anti-IL-6 receptor monoclonal antibody called tocilizumab inhibits the IL-6 cytokine signaling cascade downstream. Early in the pandemic, there was inquisitiveness in its possible application because many individuals with severe COVID-19 were found to have elevated acute phase reactant levels, including ferritin and C-reactive protein (CRP). 19,162 | RECOVERY 162 | In a UK trial, hospitalized patients with elevated CRP above 75 mg/L and hypoxaemia were randomized to receive tocilizumab rather than conventional regular medical treatment. When compared to the usual course of treatment, the tocilizumab group’s mortality was significantly lower (31% versus 35%, р = 0.0028). 162 | Proposed that patients needing mechanical ventilation, high-flow nasal cannulas, non-invasive ventilation, ECMO (extracorporeal membrane oxygenation), or patients on traditional oxygen who have drastic elevating oxygen requirements and signs of systemic inflammation (such as raised CRP) are prescribed tocilizumab in conjunction with dexamethasone. If tocilizumab is not accessible, sarilumab is advised as a backup. 19,133,154 | |
| REMAP-CAP 163 | Randomized people with extreme COVID-19 who were referred to the critical care unit within 24 hours to receive either standard therapy (dexamethasone alone) or tocilizumab together with sarilumab (an additional human anti-IL-6 receptor monoclonal antibodies) and dexamethasone. The period of organ support was shortened, and the 28-day all-cause death rates was decreased in the tocilizumab and sarilumab groups. 163 | ||||||
| Janus kinase ( JAK) inhibitor | Small molecule (oral) 152 | Individuals admitted to the hospital in need of extra oxygen, invasive or noninvasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). 19,152 | Baricitinib is a Janus kinase (JAK) blocker that prevents the synthesis of cytokines, such as IL-6, by inhibiting the stimulation of the STAT (signal transducers and activating agents of transcription) pathway. The FDA has approved baricitinib for the treatment of rheumatologic conditions, including rheumatoid arthritis. 19,164 | COV-BARRIER 164 | The death rate was lower for hospitalized individuals randomly assigned to receive baricitinib along with to their normal medication (that included dexamethasone) than for those who received a placebo. 164 | It is recommended to combine baricitinib with dexamethasone for the patients requiring ECMO, noninvasive ventilation, mechanical ventilation, high-flow nasal cannulas, or patients receiving traditional oxygen who have evidence of systemic inflammation (such as elevated CRP) along with substantially increasing oxygen demand. 19,133,154 | |
| RECOVERY 165 | Patients hospitalized with COVID-19 who received baricitinib instead of conventional therapy alone experienced a decrease in 28-day all-cause death rates (12% vs 14%, р = 0.028) in an open-label platform study. 165 |
Vaccines Available for the Prevention of COVID-19
The development of treatment options and preventative vaccinations against SARS-CoV-2 has garnered international attention since the pandemic’s onset and the disease’s effects. Prophylactic vaccinations were intended to produce protective immunity in opposition to SARS-CoV-2, whereas the treatment goals were whereas the treatment goals were centred around measures that might reduce hospitalization time and improve the survival rate of infected patients. The development of effective preventive vaccinations in opposition to SARS-CoV-2 was crucial because of the urgent global outbreak situation and its related repercussions, including limited hospital capacity and ventilators.166–168 The process of developing vaccines is lengthy and intricate, and it might take years or even decades to generate a vaccine that proves effective. On the other hand, the COVID-19 vaccine development effort took a year and was an international triumph. 169,170 Developing a COVID-19 vaccine within the allotted 12-to 24-month timeframe posed an immense challenge for the researchers.170,171 A number of factors contributed to the immediate development of the COVID-19 vaccine, including the timely release of the viral genome sequence, technological advancements and innovation, active participation from the international scientific community, a strong regulatory framework, sufficient government funding, and high market demand. Researchers from all around the world reported outstanding progress in the development of vaccines by the beginning of December 2020. On December 2, 2020, a vaccine developed by Pfizer in partnership with BioNTech, a German biotech company became the first completely tested vaccine to be authorized for use in an emergency. 170 Global vaccination campaigns have primarily employed five types of COVID-19 vaccines: protein subunit, replicating and non-replicating viral vector, nucleic acid (DNA and RNA), inactivated complete virus, and virus-like particles (VLPs) vaccines. In (Table 4), the most recent COVID-19 vaccines updates are presented with adverse effects as well as their efficacy and coverage versus various SARS-Cov-2 variants.166,167,170
 |
Table 4: The Comprehensive List of Vaccines for COVID-19 are presented with their Adverse Effects, Effectiveness, and Efficacy against several SARS-Cov-2 variants. |
Conclusion
Five years have passed since Wuhan, China, reported the initial COVID-19 occurrences. Worldwide, more than 775 million people have been infected with SARS-CoV-2, while more than 7 million of those cases have led to COVID-19 infection-related deaths. Every aspect of the socioeconomic framework has been significantly impacted by the COVID-19 outbreak, but it has especially highlighted inadequacies in the healthcare system. It demonstrated the futile struggle the world was in against the biggest threat of the twenty-first century. Infection with COVID-19 has been associated with complications in several organs, including cardiovascular, respiratory, the renal system, and gastrointestinal tract, haematological, cognitive, and dermatological issues. The comorbidities have a strong relationship with the mortality rate of COVID-19 patients. Acute instances and higher death rates are linked to COVID-19 individuals, and they are more common in people over 70 who also have underlying comorbidities. Individuals with cardiovascular illnesses had a threefold greater risk of developing an acute illness or requiring an intensive care unit (ICU) admission, compared to patients with hypertension or diabetes mellitus. Additionally, SARS-CoV-2 is recognized to have exceptional neurotropic potential. Numerous neurotoxins that cause neurological symptoms are released during the cytokine storm, which affects both the lungs and the central nervous system. In an effort to control COVID-19-related death rates and pathologies during these difficult years, a number of treatment alternatives and preventative measures, such as the COVID-19 vaccine, were considered. Since the pandemic began, there has been a tremendous evolution in the field of scientific study on COVID-19 disease treatment. Even those who have received the SARS-CoV-2 vaccination are susceptible of contracting a mild-to-moderate illness; nevertheless, effective antiviral therapy is currently available for early administration. The frequent emergence of variations resistant to existing products limits the usage of monoclonal antibodies. The management of severe and critical diseases requires the combination of immunomodulatory therapy and antiviral therapy. It is anticipated that future developments may benefit difficult-to-treat populations that are immunocompromised or have coexisting comorbidity conditions that limit the use of currently available choices. These patients may need individualized care in terms of the selection, combination, and duration of antiviral medications.
Acknowledgement
I gratefully acknowledge Servier Medical Art (https://smart.servier.com/) for providing free access to their high-quality professional images [licensed under CC BY 4.0 (https://creativecommons.org/ licenses/by/ 4.0/)], which greatly contributed to the visual clarity and quality of this publication. Servier Medical Art’s generous support is instrumental in enhancing the advancement of scientific communication.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Permission to reproduce material from other sources
Not Applicable
Author Contributions
The sole author was responsible for the conceptualization, methodology, data collection, analysis, writing, and final approval of the manuscript.
References
- Kabir MT, Uddin MS, Hossain MF, et al. nCOVID-19 Pandemic: From Molecular Pathogenesis to Potential Investigational Therapeutics. Front Cell Dev Biol. 2020;8:616. doi:10.3389/fcell.2020.00616
CrossRef - Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91-98. doi:10.1016/j.jare.2020.03.005
CrossRef - Chan-Yeung M, Xu RH. SARS: epidemiology. Respirology. 2003;8 Suppl:S9-14. doi:10.1046/j.1440-1843.2003.00518.x
CrossRef - Cheke RS, Shinde S, Ambhore J, Adhao V, Cheke D. Coronavirus: Hotspot on coronavirus disease 2019 in India. Indian J Med Sci. 2020;72(1):29-34. doi:10.25259/IJMS_33_2020
CrossRef - Peiris JSM, Lai ST, Poon LLM, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361(9366):1319-1325. doi:10.1016/s0140-6736(03)13077-2
CrossRef - World Health Organization (WHO) WHO. World Health Organization (WHO). WHO MERS Global Summary and Assessment of Risk – July 2019. 2019. Accessed June 30, 2022. https://www.who.int/publications-detail-redirect/10665-326126
- Al-Osail AM, Al-Wazzah MJ. The history and epidemiology of Middle East respiratory syndrome corona virus. Multidisciplinary Respiratory Medicine. 2017;12(1):20. doi:10.1186/s40248-017-0101-8
CrossRef - Salva EP, Villarama JB, Lopez EB, et al. Epidemiological and clinical characteristics of patients with suspected COVID-19 admitted in Metro Manila, Philippines. Tropical Medicine and Health. 2020;48(1):51. doi:10.1186/s41182-020-00241-8
CrossRef - Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. doi:10.1056/NEJMoa2001017
CrossRef - Li H, Liu Z, Ge J. Scientific research progress of COVID-19/SARS-CoV-2 in the first five months. J Cell Mol Med. 2020;24(12):6558-6570. doi:10.1111/jcmm.15364
CrossRef - Zhao S, Lin Q, Ran J, et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214-217. doi:10.1016/j.ijid.2020.01.050
CrossRef - Mungroo MR, Khan NA, Siddiqui R. Novel Coronavirus: Current Understanding of Clinical Features, Diagnosis, Pathogenesis, and Treatment Options. Pathogens. 2020;9(4):E297. doi:10.3390/pathogens9040297
CrossRef - Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. doi:10.1016/S0140-6736(20)30183-5
CrossRef - Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304-377. doi:10.1007/s00134-017-4683-6
CrossRef - Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel Coronavirus Infection in Hospitalized Infants Under 1 Year of Age in China. JAMA. 2020;323(13):1313-1314. doi:10.1001/jama.2020.2131
CrossRef - Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95. doi:10.1016/j.ijid.2020.03.017
CrossRef - Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531-538. doi:10.1007/s00392-020-01626-9
CrossRef - Ghasemiyeh P, Mohammadi-Samani S. Lessons we learned during the past four challenging years in the COVID-19 era: pharmacotherapy, long COVID complications, and vaccine development. Virol J. 2024;21(1):98. doi:10.1186/s12985-024-02370-6
CrossRef - Andrews HS, Herman JD, Gandhi RT. Treatments for COVID-19. Annual Review of Medicine. 2024;75(Volume 75, 2024):145-157. doi:10.1146/annurev-med-052422-020316
CrossRef - Elseidy SA, Awad AK, Vorla M, et al. Cardiovascular complications in the Post-Acute COVID-19 syndrome (PACS). Int J Cardiol Heart Vasc. 2022;40:101012. doi:10.1016/j.ijcha.2022.101012
CrossRef - Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536-544. doi:10.1038/s41564-020-0695-z
CrossRef - Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA. Insights into the Recent 2019 Novel Coronavirus (SARS-CoV-2) in Light of Past Human Coronavirus Outbreaks. Pathogens. 2020;9(3):E186. doi:10.3390/pathogens9030186
CrossRef - Woo PCY, Huang Y, Lau SKP, Yuen KY. Coronavirus Genomics and Bioinformatics Analysis. Viruses. 2010;2(8):1804-1820. doi:10.3390/v2081803
CrossRef - Forni D, Cagliani R, Clerici M, Sironi M. Molecular Evolution of Human Coronavirus Genomes. Trends Microbiol. 2017;25(1):35-48. doi:10.1016/j.tim.2016.09.001
CrossRef - Lim YX, Ng YL, Tam JP, Liu DX. Human Coronaviruses: A Review of Virus–Host Interactions. Diseases. 2016;4(3):26. doi:10.3390/diseases4030026
CrossRef - Miłek J, Blicharz-Domańska K. Coronaviruses in Avian Species – Review with Focus on Epidemiology and Diagnosis in Wild Birds. J Vet Res. 2018;62(3):249-255. doi:10.2478/jvetres-2018-0035
CrossRef - Su S, Wong G, Shi W, et al. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24(6):490-502. doi:10.1016/j.tim.2016.03.003
CrossRef - Boopathi S, Poma AB, Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. Journal of Biomolecular Structure and Dynamics. 2021;39(9):3409-3418. doi:10.1080/07391102.2020.1758788
CrossRef - Gupta R, Hussain A, Misra A. Diabetes and COVID-19: evidence, current status and unanswered research questions. Eur J Clin Nutr. 2020;74(6):864-870. doi:10.1038/s41430-020-0652-1
CrossRef - Korber B, Fischer WM, Gnanakaran S, et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020;182(4):812-827.e19. doi:10.1016/j.cell.2020.06.043
CrossRef - Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In: StatPearls. StatPearls Publishing; 2024. Accessed September 26, 2024. http://www.ncbi.nlm.nih.gov/books/NBK554776/
- International Committee on Taxonomy of Viruses: ICTV. Accessed June 15, 2025. https://ictv.global/
- SMART. Servier Medical Art. Accessed June 15, 2025. https://smart.servier.com/
- Andre M, Lau LS, Pokharel MD, et al. From Alpha to Omicron: How Different Variants of Concern of the SARS-Coronavirus-2 Impacted the World. Biology (Basel). 2023;12(9):1267. doi:10.3390/biology12091267
CrossRef - Davis-Gardner ME, Lai L, Wali B, et al. Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA Bivalent Booster. N Engl J Med. 2023;388(2):183-185. doi:10.1056/NEJMc2214293
CrossRef - Hoteit R, Yassine HM. Biological Properties of SARS-CoV-2 Variants: Epidemiological Impact and Clinical Consequences. Vaccines (Basel). 2022;10(6):919. doi:10.3390/vaccines10060919
CrossRef - Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631-637. doi:10.1002/path.1570
CrossRef - Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112-116. doi:10.1038/nature03712
CrossRef - Imai Y, Kuba K, Penninger JM. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp Physiol. 2008;93(5):543-548. doi:10.1113/expphysiol.2007.040048
CrossRef - Jia HP, Look DC, Shi L, et al. ACE2 Receptor Expression and Severe Acute Respiratory Syndrome Coronavirus Infection Depend on Differentiation of Human Airway Epithelia. J Virol. 2005;79(23):14614-14621. doi:10.1128/JVI.79.23.14614-14621.2005
CrossRef - Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875-879. doi:10.1038/nm1267
CrossRef - Kuba K, Imai Y, Penninger JM. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006;6(3):271-276. doi:10.1016/j.coph.2006.03.001
CrossRef - Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. doi:10.1038/s41577-020-0311-8
CrossRef - Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281-292.e6. doi:10.1016/j.cell.2020.02.058
CrossRef - Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi:10.1038/s41368-020-0074-x
CrossRef - Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420-422. doi:10.1016/S2213-2600(20)30076-X
CrossRef - Babcock GJ, Esshaki DJ, Thomas WD, Ambrosino DM. Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor. J Virol. 2004;78(9):4552-4560. doi:10.1128/jvi.78.9.4552-4560.2004
CrossRef - Wong SK, Li W, Moore MJ, Choe H, Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279(5):3197-3201. doi:10.1074/jbc.C300520200
CrossRef - Xiao X, Chakraborti S, Dimitrov AS, Gramatikoff K, Dimitrov DS. The SARS-CoV S glycoprotein: expression and functional characterization. Biochem Biophys Res Commun. 2003;312(4):1159-1164. doi:10.1016/j.bbrc.2003.11.054
CrossRef - Bosch BJ, Martina BEE, Van Der Zee R, et al. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc Natl Acad Sci U S A. 2004;101(22):8455-8460. doi:10.1073/pnas.0400576101
CrossRef - Liu S, Xiao G, Chen Y, et al. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet. 2004;363(9413):938-947. doi:10.1016/S0140-6736(04)15788-7
CrossRef - Simmons G, Gosalia DN, Rennekamp AJ, Reeves JD, Diamond SL, Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci U S A. 2005;102(33):11876-11881. doi:10.1073/pnas.0505577102
CrossRef - Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. Published online February 17, 2020:S0006-291X(20)30339-9. doi:10.1016/j.bbrc.2020.02.071
CrossRef - Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260-1263. doi:10.1126/science.abb2507
CrossRef - Eastman RT, Roth JS, Brimacombe KR, et al. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent Sci. 2020;6(5):672-683. doi:10.1021/acscentsci.0c00489
CrossRef - Masters PS. The molecular biology of coronaviruses. Adv Virus Res. 2006;66:193-292. doi:10.1016/S0065-3527(06)66005-3
CrossRef - Cameron MJ, Bermejo-Martin JF, Danesh A, Muller MP, Kelvin DJ. Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res. 2008;133(1):13-19. doi:10.1016/j.virusres.2007.02.014
CrossRef - Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19(2):181-193. doi:10.1016/j.chom.2016.01.007
CrossRef - Williams AE, Chambers RC. The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol. 2014;306(3):L217-230. doi:10.1152/ajplung.00311.2013
CrossRef - Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270-273. doi:10.1038/s41586-020-2012-7
CrossRef - Dasgupta A, Bakshi A, Mukherjee S, et al. Epidemiological Challenges in Pandemic Coronavirus Disease (COVID-19): Role of Artificial Intelligence. Published online May 14, 2020. doi:10.20944/preprints202005.0234.v1
CrossRef - Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. doi:10.1186/s40249-020-00646-x
CrossRef - Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3). doi:10.2807/1560-7917.ES.2020.25.3.2000045
CrossRef - Dhamad AE, Rhida MAA. COVID-19: molecular and serological detection methods. PeerJ. 2020;8:e10180. doi:10.7717/peerj.10180
CrossRef - Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA. 2020;323(22):2249-2251. doi:10.1001/jama.2020.8259
CrossRef - Mishra A, Nair N, Tripathi V, Pathak Y, Majeed J. History, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19). Coronaviruses. 2022;3(1):65-72. doi:10.2174/26667967026662 10805101958
CrossRef - Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26(7):1033-1036. doi:10.1038/s41591-020-0913-5
CrossRef - Pavlova IP, Nair SS, Kyprianou N, Tewari AK. The Rapid Coronavirus Antibody Test: Can We Improve Accuracy? Frontiers in Medicine. 2020;7. Accessed July 15, 2022. https://www.frontiersin.org/articles/ 10.3389/fmed.2020.00569
CrossRef - Petherick A. Developing antibody tests for SARS-CoV-2. Lancet. 2020;395(10230):1101-1102. doi:10.1016/S0140-6736(20)30788-1
CrossRef - Tang YW, Schmitz JE, Persing DH, Stratton CW. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J Clin Microbiol. 2020;58(6):e00512-20. doi:10.1128/JCM.00512-20
CrossRef - Whitman JD, Hiatt J, Mowery CT, et al. Test performance evaluation of SARS-CoV-2 serological assays. medRxiv. Published online May 17, 2020:2020.04.25.20074856. doi:10.1101/2020.04.25.20074856
CrossRef - Augustine R, Das S, Hasan A, et al. Rapid Antibody-Based COVID-19 Mass Surveillance: Relevance, Challenges, and Prospects in a Pandemic and Post-Pandemic World. Journal of Clinical Medicine. 2020;9(10):3372. doi:10.3390/jcm9103372
CrossRef - Li Z, Yi Y, Luo X, et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92(9):1518-1524. doi:10.1002/jmv.25727
CrossRef - Sheikhi K, Shirzadfar H, Sheikhi M. A review on novel coronavirus (Covid-19): symptoms, transmission and diagnosis tests. Research in Infectious Diseases and Tropical Medicine. 2020;2(1):1-8. doi:http://dx.doi.org/10.33702/ridtm.2020.2.1.1
- Guan W jie, Ni Z yi, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine. 2020;382(18):1708-1720. doi:10.1056/NEJMoa2002032
CrossRef - Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. doi:10.1016/j.diabres.2020.108142
CrossRef - Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens. 2020;33(5):373-374. doi:10.1093/ajh/hpaa057
CrossRef - Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242. doi:10.1001/jama.2020.2648
CrossRef - Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585
CrossRef - Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiology. 2020;5(7):831-840. doi:10.1001/jamacardio. 2020.1286
CrossRef - Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145-151. doi:10.3760/cma.j.issn.0254-6450.2020.02.003
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
CrossRef - Ruan Q, Yang K, Wang W, Jiang L, Song J. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(6):1294-1297. doi:10.1007/s00134-020-06028-z
CrossRef - Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J. 2021;42(2):206. doi:10.1093/eurheartj/ehaa190
CrossRef - Inciardi RM, Lupi L, Zaccone G, et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):819-824. doi:10.1001/jamacardio.2020.1096
CrossRef - Guo T, Fan Y, Chen M, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiology. 2020;5(7):811-818. doi:10.1001/jamacardio.2020.1017
CrossRef - Shi S, Qin M, Shen B, et al. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802-810. doi:10.1001/jamacardio.2020.0950
CrossRef - Drucker DJ. Coronavirus Infections and Type 2 Diabetes-Shared Pathways with Therapeutic Implications. Endocr Rev. 2020;41(3):bnaa011. doi:10.1210/endrev/bnaa011
CrossRef - Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;323(18):1775-1776. doi:10.1001/jama.2020.4683
CrossRef - Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574-1581. doi:10.1001/jama.2020.5394
CrossRef - Dunn EJ, Grant PJ. Type 2 diabetes: an atherothrombotic syndrome. Curr Mol Med. 2005;5(3):323-332. doi:10.2174/1566524053766059
CrossRef - Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi:10.1016/S0140-6736(20)30566-3
CrossRef - Guan W jie, Liang W hua, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi:10.1183/13993003.00547-2020
CrossRef - Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. doi:10.1016/S2213-2600(20)30079-5
CrossRef - Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Annals of Clinical and Translational Neurology. 2020;7(11):2221-2230. doi:10.1002/acn3.51210
CrossRef - Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurology. 2020;77(6):683-690. doi:10.1001/ jamaneurol.2020.1127
CrossRef - Brola W, Wilski M. Neurological consequences of COVID-19. Pharmacol Rep. 2022;74(6):1208-1222. doi:10.1007/s43440-022-00424-6
CrossRef - Berlit P, Bösel J, Gahn G, et al. “Neurological manifestations of COVID-19” – guideline of the German society of neurology. Neurol Res Pract. 2020;2(1):51. doi:10.1186/s42466-020-00097-7
CrossRef - Majolo F, Silva GL da, Vieira L, et al. Neuropsychiatric Disorders and COVID-19: What We Know So Far. Pharmaceuticals. 2021;14(9):933. doi:10.3390/ph14090933
CrossRef - Mahboubi Mehrabani M, Karvandi MS, Maafi P, Doroudian M. Neurological complications associated with Covid-19; molecular mechanisms and therapeutic approaches. Reviews in Medical Virology. 2022;32(6):e2334. doi:10.1002/rmv.2334
CrossRef - Wenting A, Gruters A, van Os Y, et al. COVID-19 Neurological Manifestations and Underlying Mechanisms: A Scoping Review. Front Psychiatry. 2020;11. doi:10.3389/fpsyt.2020.00860
CrossRef - Stilhano RS, Costa AJ, Nishino MS, et al. SARS‐CoV‐2 and the possible connection to ERs, ACE2, and RAGE: Focus on susceptibility factors. FASEB J. 2020;34(11):14103-14119. doi:10.1096/fj.202001394RR
CrossRef - Abboud H, Abboud FZ, Kharbouch H, Arkha Y, El Abbadi N, El Ouahabi A. COVID-19 and SARS-Cov-2 Infection: Pathophysiology and Clinical Effects on the Nervous System. World Neurosurgery. 2020;140:49-53. doi:10.1016/j.wneu.2020.05.193
CrossRef - Azim D, Nasim S, Kumar S, et al. Neurological Consequences of 2019-nCoV Infection: A Comprehensive Literature Review. Cureus. 2020;12. doi:10.7759/cureus.8790
CrossRef - Wang Z, Yang Y, Liang X, et al. COVID-19 Associated Ischemic Stroke and Hemorrhagic Stroke: Incidence, Potential Pathological Mechanism, and Management. Front Neurol. 2020;11:571996. doi:10.3389/fneur.2020.571996
CrossRef - Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain, Behavior, and Immunity. 2020;87:18-22. doi:10.1016/j.bbi.2020.03.031
CrossRef - Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7:11. doi:10.1186/s40779-020-00240-0
CrossRef - Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251-2261. doi:10.1007/s00405-020-05965-1
CrossRef - Saniasiaya J, Islam MA, Abdullah B. Prevalence of Olfactory Dysfunction in Coronavirus Disease 2019 (COVID-19): A Meta-analysis of 27,492 Patients. The Laryngoscope. 2021;131(4):865-878. doi:10.1002/lary.29286
CrossRef - Orsucci D, Ienco EC, Nocita G, Napolitano A, Vista M. Neurological features of COVID-19 and their treatment: a review. Drugs Context. 2020;9:2020-5-1. doi:10.7573/dic.2020-5-1
CrossRef - Boesveldt S, Postma EM, Boak D, et al. Anosmia—A Clinical Review. Chemical Senses. 2017;42(7):513-523. doi:10.1093/chemse/bjx025
CrossRef - Pun BT, Badenes R, Calle GHL, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. The Lancet Respiratory Medicine. 2021;9(3):239-250. doi:10.1016/S2213-2600(20)30552-X
CrossRef - Shah VA, Nalleballe K, Zaghlouleh ME, Onteddu S. Acute encephalopathy is associated with worse outcomes in COVID-19 patients. Brain, Behavior, & Immunity – Health. 2020;8:100136. doi:10.1016/j.bbih.2020.100136
CrossRef - Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. New England Journal of Medicine. 2020;382(20):e60. doi:10.1056/NEJMc2009787
CrossRef - Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889-891. doi:10.1136/jnnp-2020-323586
CrossRef - Bekelis K, Missios S, Ahmad J, et al. Ischemic Stroke Occurs Less Frequently in Patients With COVID-19. Stroke. 2020;51(12):3570-3576. doi:10.1161/STROKEAHA.120.031217
CrossRef - Qureshi AI, Baskett WI, Huang W, et al. Acute Ischemic Stroke and COVID-19. Stroke. 2021;52(3):905-912. doi:10.1161/STROKEAHA.120.031786
CrossRef - Klok FA, Kruip MJHA, Meer NJM van der, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research. 2020;191:145-147. doi:10.1016/j.thromres.2020.04.013
CrossRef - Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thrombosis Research. 2020;191:9-14. doi:10.1016/j.thromres.2020.04.024
CrossRef - Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral Venous Sinus Thrombosis as a Presentation of COVID-19. European Journal of Case Reports in Internal Medicine. Published online April 29, 2020. doi:10.12890/2020_001691
CrossRef - Tu TM, Goh C, Tan YK, et al. Cerebral Venous Thrombosis in Patients with COVID-19 Infection: a Case Series and Systematic Review. Journal of Stroke and Cerebrovascular Diseases. 2020;29(12). doi:10.1016/j.jstrokecerebrovasdis.2020.105379
CrossRef - Khatana SAM, Groeneveld PW. Health Disparities and the Coronavirus Disease 2019 (COVID-19) Pandemic in the USA. J GEN INTERN MED. 2020;35(8):2431-2432. doi:10.1007/s11606-020-05916-w
CrossRef - Baldini T, Asioli GM, Romoli M, et al. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: A systematic review and meta-analysis. European Journal of Neurology. 2021;28(10):3478-3490. doi:10.1111/ene.14727
CrossRef - Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Connolly AMF. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. The Lancet. 2021;398(10300):599-607. doi:10.1016/S0140-6736(21)00896-5
CrossRef - Katz JM, Libman RB, Wang JJ, et al. Cerebrovascular Complications of COVID-19. Stroke. 2020;51(9):e227-e231. doi:10.1161/STROKEAHA.120.031265
CrossRef - Fridman S, Bres Bullrich M, Jimenez-Ruiz A, et al. Stroke risk, phenotypes, and death in COVID-19. Neurology. 2020;95(24):e3373-e3385. doi:10.1212/WNL.0000000000010851
CrossRef - Jin M, Tong Q. Rhabdomyolysis as Potential Late Complication Associated with COVID-19 – Volume 26, Number 7—July 2020 – Emerging Infectious Diseases journal – CDC. Published online 2020. doi:10.3201/eid2607.200445
CrossRef - Restivo DA, Centonze D, Alesina A, Marchese-Ragona R. Myasthenia Gravis Associated With SARS-CoV-2 Infection. Ann Intern Med. 2020;173(12):1027-1028. doi:10.7326/L20-0845
CrossRef - Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021;144(2):682-693. doi:10.1093/brain/awaa433
CrossRef - Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? The Lancet Neurology. 2020;19(5):383-384. doi:10.1016/S1474-4422(20)30109-5
CrossRef - Toscano G, Palmerini F, Ravaglia S, et al. Guillain–Barré Syndrome Associated with SARS-CoV-2. New England Journal of Medicine. 2020;382(26):2574-2576. doi:10.1056/NEJMc2009191
CrossRef - Luijten LWG, Leonhard SE, van der Eijk AA, et al. Guillain-Barré syndrome after SARS-CoV-2 infection in an international prospective cohort study. Brain. 2021;144(11):3392-3404. doi:10.1093/brain/awab279
CrossRef - (NIH) NI of H. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Accessed September 27, 2024. https://www.covid19treatmentguidelines.nih.gov/.
- Gandhi RT, Lynch JB, Del Rio C. Mild or Moderate Covid-19. N Engl J Med. 2020;383(18):1757-1766. doi:10.1056/NEJMcp2009249
CrossRef - Lui G, Guaraldi G. Drug treatment of COVID-19 infection. Curr Opin Pulm Med. 2023;29(3):174-183. doi:10.1097/MCP.0000000000000953
CrossRef - Lim S, Tignanelli CJ, Hoertel N, Boulware DR, Usher MG. Prevalence of Medical Contraindications to Nirmatrelvir/Ritonavir in a Cohort of Hospitalized and Nonhospitalized Patients With COVID-19. Open Forum Infect Dis. 2022;9(8):ofac389. doi:10.1093/ofid/ofac389
CrossRef - Shah MM, Joyce B, Plumb ID, et al. Paxlovid Associated with Decreased Hospitalization Rate Among Adults with COVID-19 – United States, April-September 2022. MMWR Morb Mortal Wkly Rep. 2022;71(48):1531-1537. doi:10.15585/mmwr.mm7148e2
CrossRef - Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med. 2022;386(15):1397-1408. doi:10.1056/NEJMoa2118542
CrossRef - Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022;386(6):509-520. doi:10.1056/NEJMoa2116044
CrossRef - Suzuki Y, Shibata Y, Minemura H, et al. Real-world clinical outcomes of treatment with molnupiravir for patients with mild-to-moderate coronavirus disease 2019 during the Omicron variant pandemic. Clin Exp Med. 2023;23(6):2715-2723. doi:10.1007/s10238-022-00949-3
CrossRef - Wai AKC, Chan CY, Cheung AWL, et al. Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg Health West Pac. 2023;30:100602. doi:10.1016/j.lanwpc.2022.100602
CrossRef - Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22(12):1681-1693. doi:10.1016/S1473-3099(22)00507-2
CrossRef - Bartoletti M, Azap O, Barac A, et al. ESCMID COVID-19 living guidelines: drug treatment and clinical management. Clin Microbiol Infect. 2022;28(2):222-238. doi:10.1016/j.cmi.2021.11.007
CrossRef - RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693-704. doi:10.1056/NEJMoa2021436
CrossRef - Wang Q, Iketani S, Li Z, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186(2):279-286.e8. doi:10.1016/j.cell.2022.12.018
CrossRef - Connors JM, Iba T, Gandhi RT. Thrombosis and COVID-19: Controversies and (Tentative) Conclusions. Clin Infect Dis. Published online February 4, 2021:ciab096. doi:10.1093/cid/ciab096
CrossRef - Spyropoulos AC, Bonaca MP. Studying the coagulopathy of COVID-19. Lancet. 2022;399(10320):118-119. doi:10.1016/S0140-6736(21)01906-1
CrossRef - Barco S, Voci D, Held U, et al. Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet Haematol. 2022;9(8):e585-e593. doi:10.1016/S2352-3026(22)00175-2
CrossRef - Cools F, Virdone S, Sawhney J, et al. Thromboprophylactic low-molecular-weight heparin versus standard of care in unvaccinated, at-risk outpatients with COVID-19 (ETHIC): an open-label, multicentre, randomised, controlled, phase 3b trial. Lancet Haematol. 2022;9(8):e594-e604. doi:10.1016/S2352-3026(22)00173-9
CrossRef - ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators, et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med. 2021;385(9):790-802. doi:10.1056/NEJMoa2105911
CrossRef - REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators, et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med. 2021;385(9):777-789. doi:10.1056/NEJMoa2103417
CrossRef - Li G, Hilgenfeld R, Whitley R, De Clercq E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat Rev Drug Discov. 2023;22(6):449-475. doi:10.1038/s41573-023-00672-y
CrossRef - Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 – Final Report. N Engl J Med. 2020;383(19):1813-1826. doi:10.1056/NEJMoa2007764
CrossRef - Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network – United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993-998.
doi:10.15585/mmwr.mm6930e1
CrossRef - Ali K, Azher T, Baqi M, et al. Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial. CMAJ. 2022;194(7):E242-E251. doi:10.1503/cmaj.211698
CrossRef - WHO Solidarity Trial Consortium. Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet. 2022;399(10339):1941-1953. doi:10.1016/S0140-6736(22)00519-0
CrossRef - Gottlieb RL, Vaca CE, Paredes R, et al. Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients. N Engl J Med. 2022;386(4):305-315. doi:10.1056/NEJMoa2116846
CrossRef - Pfizer. Pfizer Reports Additional Data on PAXLOVIDTM Supporting Upcoming New Drug Application Submission to U.S. FDA | Pfizer. Pfizer. Accessed September 27, 2024.https://www.pfizer.com/news/ press-release/ press-release-detail/pfizer-reports-additional-data-paxlovidtm-supporting
- Ganatra S, Dani SS, Ahmad J, et al. Oral Nirmatrelvir and Ritonavir in Nonhospitalized Vaccinated Patients With Coronavirus Disease 2019. Clin Infect Dis. 2023;76(4):563-572. doi:10.1093/cid/ciac673
CrossRef - Butler CC, Hobbs FDR, Gbinigie OA, et al. Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial. Lancet. 2023;401(10373):281-293. doi:10.1016/S0140-6736(22)02597-1
CrossRef - RECOVERY Collaborative Group. Electronic address: recoverytrial@ndph.ox.ac.uk, RECOVERY Collaborative Group. Higher dose corticosteroids in patients admitted to hospital with COVID-19 who are hypoxic but not requiring ventilatory support (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2023;401(10387):1499-1507. doi:10.1016/S0140-6736(23)00510-X
CrossRef - RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397(10285):1637-1645. doi:10.1016/S0140-6736(21)00676-0
CrossRef - REMAP-CAP Investigators, Gordon AC, Mouncey PR, et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384(16):1491-1502. doi:10.1056/NEJMoa2100433
CrossRef - Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407-1418. doi:10.1016/S2213-2600(21)00331-3
CrossRef - RECOVERY Collaborative Group. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet. 2022;400(10349):359-368. doi:10.1016/S0140-6736(22)01109-6
CrossRef - Firouzabadi N, Ghasemiyeh P, Moradishooli F, Mohammadi-Samani S. Update on the effectiveness of COVID-19 vaccines on different variants of SARS-CoV-2. Int Immunopharmacol. 2023;117:109968. doi:10.1016/j.intimp.2023.109968
CrossRef - Ghasemiyeh P, Mohammadi-Samani S, Firouzabadi N, Dehshahri A, Vazin A. A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. Int Immunopharmacol. 2021;100:108162. doi:10.1016/j.intimp.2021.108162
CrossRef - Zarkesh K, Entezar-Almahdi E, Ghasemiyeh P, et al. Drug-based therapeutic strategies for COVID-19-infected patients and their challenges. Future Microbiol. Published online 2021:10.2217/fmb-2021-0116. doi:10.2217/fmb-2021-0116
CrossRef - Haurat MF, Aduse-Opoku J, Rangarajan M, et al. Selective Sorting of Cargo Proteins into Bacterial Membrane Vesicles. J Biol Chem. 2011;286(2):1269-1276. doi:10.1074/jbc.M110.185744
CrossRef - Jha DK, Pranay K, Samiksha, Kumar A, Yashvardhini N. The status of COVID-19 vaccines in India: A review. Vacunas. Published online April 25, 2023. doi:10.1016/j.vacun.2023.04.003
CrossRef - Kashte S, Gulbake A, El-Amin III SF, Gupta A. COVID-19 vaccines: rapid development, implications, challenges and future prospects. Hum Cell. 2021;34(3):711-733. doi:10.1007/s13577-021-00512-4
CrossRef - Lamb YN. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs. 2021;81(4):495-501. doi:10.1007/s40265-021-01480-7
CrossRef - Bassi J, Giannini O, Silacci-Fregni C, et al. Poor neutralization and rapid decay of antibodies to SARS-CoV-2 variants in vaccinated dialysis patients. PLoS ONE. 2022;17(2). doi:10.1371/journal.pone.0263328
CrossRef - Del Rio C, Malani PN, Omer SB. Confronting the Delta Variant of SARS-CoV-2, Summer 2021. JAMA. 2021;326(11):1001-1002. doi:10.1001/jama.2021.14811
CrossRef - Sánchez-Sendra B, Albert E, Zulaica J, et al. Neutralizing antibodies against SARS-CoV-2 variants of concern elicited by the comirnaty COVID-19 vaccine in nursing home residents. Sci Rep. 2022;12:3788. doi:10.1038/s41598-022-07849-2
CrossRef - Sheikh A, Robertson C, Taylor B. BNT162b2 and ChAdOx1 nCoV-19 Vaccine Effectiveness against Death from the Delta Variant. The New England Journal of Medicine. doi:10.1056/NEJMc2113864
CrossRef - T T, H Z, Mi S, et al. Comparison of Neutralizing Antibody Titers Elicited by mRNA and Adenoviral Vector Vaccine against SARS-CoV-2 Variants. bioRxiv : the preprint server for biology. Published online August 6, 2021. doi:10.1101/2021.07.19.452771
CrossRef - Thiagarajan K. What do we know about India’s Covaxin vaccine? BMJ. 2021;373:n997. doi:10.1136/bmj.n997
CrossRef - Wilhelm A, Toptan T, Pallas C, et al. Antibody-Mediated Neutralization of Authentic SARS-CoV-2 B.1.617 Variants Harboring L452R and T478K/E484Q. Viruses. 2021;13(9):1693. doi:10.3390/v13091693
CrossRef - Park KS, Sun X, Aikins ME, Moon JJ. Non-viral COVID-19 vaccine delivery systems. Adv Drug Deliv Rev. 2021;169:137-151. doi:10.1016/j.addr.2020.12.008
CrossRef - Chagla Z. In high-risk adults, the Moderna vaccine had 94% efficacy against COVID-19 ≥14 d after the 2nd dose. Ann Intern Med. 2021;174(3):JC28. doi:10.7326/ACPJ202103160-028
CrossRef - Grannis SJ, Rowley EA, Ong TC, et al. Interim Estimates of COVID-19 Vaccine Effectiveness Against COVID-19–Associated Emergency Department or Urgent Care Clinic Encounters and Hospitalizations Among Adults During SARS-CoV-2 B.1.617.2 (Delta) Variant Predominance — Nine States, June–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(37):1291-1293. doi:10.15585/mmwr.mm7037e2
CrossRef - Pormohammad A, Zarei M, Ghorbani S, et al. Effectiveness of COVID-19 Vaccines against Delta (B.1.617.2) Variant: A Systematic Review and Meta-Analysis of Clinical Studies. Vaccines (Basel). 2021;10(1):23. doi:10.3390/vaccines10010023
CrossRef - Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979-1993. doi:10.1016/S0140-6736(20)32466-1
CrossRef - Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111. doi:10.1016/S0140-6736(20)32661-1
CrossRef - Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881-891. doi:10.1016/S0140-6736(21)00432-3
CrossRef - Griffin S. Covid-19: AstraZeneca vaccine prevents 79% of symptomatic disease and 100% of severe disease, US study finds. BMJ. 2021;372:n793. doi:10.1136/bmj.n793
CrossRef - Sadoff J, Gray G, Vandebosch A, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. Published online April 21, 2021:NEJMoa2101544. doi:10.1056/NEJMoa2101544
CrossRef - Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):202-221. doi:10.1016/j.cmi.2021.10.005
CrossRef - Sadoff J, Gray G, Vandebosch A, et al. Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S. N Engl J Med. Published online February 9, 2022:NEJMoa2117608. doi:10.1056/NEJMoa2117608
CrossRef - Tian JH, Patel N, Haupt R, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. 2021;12:372. doi:10.1038/s41467-020-20653-8
CrossRef - Heath PT, Galiza EP, Baxter DN, et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N Engl J Med. Published online June 30, 2021:NEJMoa2107659. doi:10.1056/NEJMoa2107659
CrossRef - Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. 2022;20:200. doi:10.1186/s12916-022-02397-y
CrossRef - Mallapaty S, Callaway E, Kozlov M, Ledford H, Pickrell J, Van Noorden R. How COVID vaccines shaped 2021 in eight powerful charts. Nature. 2021;600(7890):580-583. doi:10.1038/d41586-021-03686-x
CrossRef - Ranzani OT, Hitchings MDT, Dorion M, et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021;374:n2015. doi:10.1136/bmj.n2015
CrossRef - Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803-812. doi:10.1016/S1473-3099(20)30987-7
CrossRef - Ghiasi N, Valizadeh R, Arabsorkhi M, et al. Efficacy and side effects of Sputnik V, Sinopharm and AstraZeneca vaccines to stop COVID-19; a review and discussion. Immunopathol Persa. 2021;7(2):e31-e31. doi:10.34172/ipp.2021.31
CrossRef - Xia S, Duan K, Zhang Y, et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes. JAMA. 2020;324(10):1-10. doi:10.1001/jama.2020.15543
CrossRef - Ahamed F, Ganesan S, James A, Zaher WA. Understanding perception and acceptance of Sinopharm vaccine and vaccination against COVID–19 in the UAE. BMC Public Health. 2021;21:1602. doi:10.1186/s12889-021-11620-z
CrossRef - Srivastava RK, Ish P, Covid-Vaccination Group S. The initial experience of COVID-19 vaccination from a tertiary care centre of India. Monaldi Arch Chest Dis. 2021;91(4). doi:10.4081/monaldi.2021.1816
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





