Manuscript accepted on : 06-09-2025
Published online on: 18-09-2025
Plagiarism Check: Yes
Reviewed by: Dr. Sarraa Dhiaa Kasim
Second Review by: Dr. Marwa Husam
Final Approval by: Dr. Ali Elshafei
Naringenin: A Potential Flavonoid Phytochemical For Diabetes Management
Kajal Pansare* , Yogesh Ahire
, Yogesh Ahire and Vinod Bairagi
and Vinod Bairagi
Department of Pharmacology, KBHSS Trust’s, Institute of Pharmacy, Maharashtra, India
Corresponding Author E-mail:kajalgsonawane@gmail.com
ABSTRACT: Diabetes mellitus is a chronic metabolic disorder characterized by persistent hyperglycemia resulting from impaired insulin secretion, insulin resistance, or both, leading to severe complications affecting multiple organs. Current antidiabetic therapies, although effective, are often associated with limitations such as side effects, high cost, and incomplete efficacy, highlighting the need for safer and more effective alternatives. Naringenin, a naturally occurring flavonoid abundant in citrus fruits, has gained significant attention for its antidiabetic potential. Its pharmacological activities include antioxidant, anti-inflammatory, insulin-sensitizing, and glucose-regulating effects, mediated through modulation of oxidative stress, inflammatory pathways, insulin signaling, and lipid metabolism. However, clinical application of naringenin is hindered by poor bioavailability, rapid metabolism, and lack of standardized formulations. Advances in delivery systems, such as nanoparticles, liposomes, and encapsulation, alongside synergistic use with conventional therapies, show promise in overcoming these limitations. This review provides a comprehensive overview of the chemical properties, mechanisms of action, pharmacokinetics, safety, therapeutic potential, and research challenges of naringenin in diabetes management. In conclusion, naringenin represents a promising supplementary therapeutic strategy for diabetes management. With further research aimed at optimizing formulation strategies, elucidating mechanisms of action, and validating its efficacy in human clinical trials, naringenin could pave the way toward innovative and safer treatment approaches for achieving improved glycemic control and long-term metabolic health.
KEYWORDS: Anti-inflammatory; Antioxidant; Citrus fruits; Diabetes; Flavonoid; Insulin sensitization; Naringenin
| Copy the following to cite this article: Pansare K, Ahire Y, Bairagi V. Naringenin: A Potential Flavonoid Phytochemical For Diabetes Management. Biotech Res Asia 2025;22(3). |
| Copy the following to cite this URL: Pansare K, Ahire Y, Bairagi V. Naringenin: A Potential Flavonoid Phytochemical For Diabetes Management. Biotech Res Asia 2025;22(3). Available from: https://bit.ly/48kQSQe |
Introduction
Diabetes is a chronic metabolic disorder characterized by impaired insulin production or utilization, leading to persistent hyperglycaemia and progressive damage to multiple organ systems.1,2 Globally, the prevalence of diabetes has risen dramatically, from 200 million cases in 1990 to 830 million in 2022, with the steepest increase observed in low- and middle-income countries. In 2022, an estimated 14% of adults aged 18 years and older were living with diabetes, yet more than half were not receiving appropriate treatment. Diabetes and its complications, including cardiovascular disease, kidney failure, blindness, stroke, and lower-limb amputation, accounted for over 2 million deaths in 2021, with nearly half occurring before the age of 70. Unlike many other non-communicable diseases where mortality has declined, diabetes-related mortality continues to rise. Importantly, type 2 diabetes can often be prevented or delayed through lifestyle modification, and effective management strategies combining diet, physical activity, medication, and regular screening can significantly reduce complications.3,4
Currently available diabetic treatments include insulin therapy, oral hypoglycemic medications, and lifestyle changes.5 However, these treatments often come with limitations such as side effects, high costs, and the inability to prevent or reverse disease progression.6 For instance, insulin therapy can lead to weight gain and hypoglycemia, while some oral medications may cause gastrointestinal disturbances and cardiovascular issues.7,8 Therefore, there is a pressing need for more effective and safer therapeutic alternatives. The limitations of current treatments highlight the necessity for new antidiabetic treatments that are not only effective but also have fewer side effects. Natural compounds, particularly phytochemicals, have gained attention as potential alternatives due to their diverse biological activities and lower toxicity profiles.9
Plant-based foods such as fruits and vegetables are an excellent source of flavonoids, a type of polyphenolic chemicals. Their anti-inflammatory, anti-oxidant, and antidiabetic properties are widely recognized.10 Flavonoids produce their effects through several mechanisms, including the modulation of key enzymes and signaling pathways involved in glucose metabolism.11 Phytochemicals, including flavonoids, play a significant role in diabetes management due to their ability to improve insulin sensitivity, reduce oxidative stress, and modulate glucose homeostasis.12 These natural compounds offer a complementary approach to traditional diabetes treatments, with the potential to mitigate complications and improve overall health outcomes.13-16 The flavonoid naringenin is commonly found in citrus fruits such as grapefruit, orange, tomato, and orange juice.17 Its antioxidant, anti-inflammatory, and lipid-lowering properties make it a promising candidate for the management of diabetes.18
The primary aim of this review is to thoroughly assess the potential of naringenin as a therapeutic agent for diabetes management. This includes a comprehensive analysis of its mechanisms of action, pharmacokinetics, preclinical and clinical evidence, safety profile, and future research directions. The scope of the review encompasses an overview of current diabetes treatments and their limitations, the role of flavonoids in diabetes management, and a detailed examination of naringenin’s therapeutic potential.19
Chemical Structure and Properties of Naringenin
Naringenin belongs to the class of flavanones, as a flavonoid. A 15-carbon structure consisting of two phenyl rings (A and B) and one heterocyclic ring (C) characterizes its chemical structure, as shown in Fig. 1. The molecular formula of naringenin is C15H12O5. Its systematic IUPAC name is 4′,5,7-trihydroxyflavanone.20
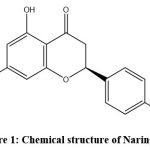 |
Figure 1: Chemical structure of Naringenin. |
Naringenin is a crystalline compound (white to light yellow) with a melting point of 250–252 °C. It is poorly soluble in water but dissolves in ethanol, methanol, and DMSO. It shows maximum absorption at ~289 nm in methanol and has pKa values of ~7.29 and 9.05, reflecting acidic hydroxyl groups. These properties influence its solubility, reactivity, and biological interactions. Its ability to form hydrogen bonds and transfer electrons underlies its antioxidant and anti-inflammatory effects.21,22 Citrus fruits (especially grapefruits, oranges, and peels), tomatoes, and herbs like oregano are the main dietary sources of naringenin. It is also available in supplemental forms such as capsules, tablets, powders, and functional foods. Concentrations vary depending on plant part, extraction method, and processing conditions.23,24 The dietary and supplemental sources of naringenin are detailed in Table 1.
Table 1: Dietary and Supplemental Sources of Naringenin
| Source Type | Examples | Form/Use | Key Notes |
| Dietary Sources | Grapefruit | Fruit & juice | Richest natural source of naringenin |
| Orange | Fruit & peel | Peel contains higher concentrations; used in culinary & medicinal applications | |
| Tomato | Fruit | Lower levels compared to citrus fruits | |
| Oregano | Herb | Contributes to dietary intake in smaller amounts | |
| Supplemental Sources | Capsules & Tablets | Oral supplements | Provide concentrated doses; easily accessible |
| Powder Form | Nutraceutical formulations | Incorporated into health supplements for enhanced benefits | |
| Functional Foods & Beverages | Fortified products | Added to boost nutritional and therapeutic value |
Pathophysiology of Diabetes Mellitus
Type 1 diabetes mellitus (T1DM) is an autoimmune disorder in which the immune system destroys pancreatic β-cells, leading to little or no insulin production. It often develops in childhood or adolescence and requires lifelong insulin therapy to regulate blood glucose and prevent complications.25 Type 2 diabetes mellitus (T2DM), the most common form (90–95% of cases), is characterized by insulin resistance and relative insulin deficiency. Risk factors include obesity, physical inactivity, and genetic susceptibility. Once considered an adult disease, it is increasingly affecting younger populations. Management involves lifestyle changes, oral hypoglycemic agents, and sometimes insulin.26 Gestational diabetes mellitus (GDM) develops during pregnancy due to glucose intolerance and increases health risks for both mother and child. It raises the likelihood of complications such as preeclampsia, macrosomia, and future T2DM. Treatment typically includes dietary adjustments, physical activity, and, if necessary, insulin or oral medications.27
Pathophysiological Mechanisms Involved
Type 1 diabetes mellitus (T1DM) is an autoimmune disorder in which the immune system destroys pancreatic β-cells, resulting in little or no insulin production. Genetic predisposition (such as HLA gene variants) and environmental triggers like viral infections contribute to its onset, and lifelong insulin therapy is required.25 Type 2 diabetes mellitus (T2DM), the most common form, is marked by insulin resistance and progressive β-cell dysfunction. It is influenced by obesity, poor diet, sedentary lifestyle, chronic inflammation, lipotoxicity, and genetic factors, and is increasingly seen in younger individuals. Oxidative stress, through excessive reactive oxygen species, impairs insulin signaling and β-cell function, while chronic low-grade inflammation driven by cytokines (e.g., CRP, IL-6, TNF-α) promotes insulin resistance and β-cell apoptosis. Additional contributors include lipotoxicity from excess free fatty acids, ectopic fat deposition in the liver and muscle, genetic susceptibility, and unhealthy lifestyle choices.28 Gestational diabetes mellitus (GDM), on the other hand, develops during pregnancy due to hormonal changes and placental factors that induce insulin resistance. Risk factors include maternal obesity, advanced age, and family history of diabetes, with GDM posing risks for both mother and child and serving as a predictor of future T2DM.29
Current Management Strategies and their Limitations
Diabetes management involves lifestyle modifications and pharmacological therapies, each with distinct benefits and drawbacks. These are summarized in Table 2. Healthy diet and regular physical activity form the cornerstone of diabetes care, improving glycemic control and overall health. However, sustaining these habits is often difficult due to personal preferences, physical limitations, or socioeconomic barriers. Insulin therapy is crucial for T1DM and advanced T2DM, but it is associated with hypoglycemia, weight gain, and lifelong dependence.30 Oral antidiabetic drugs such as metformin, sulfonylureas, DPP-4 inhibitors, and SGLT2 inhibitors improve glycemic control but may cause gastrointestinal issues, hypoglycemia, or cardiovascular risks.31 GLP-1 receptor agonists offer dual benefits of glucose regulation and cardiovascular protection, yet their high cost and gastrointestinal side effects limit use.32 Despite available options, long-term management remains challenging. High costs of insulin and newer agents hinder accessibility in low-resource settings. Complex regimens often lead to poor adherence, reducing treatment effectiveness. Furthermore, many patients still progress to complications such as neuropathy, retinopathy, and cardiovascular disease, underscoring the urgent need for safer, more affordable, and more effective therapies.33
Table 2: Current Management Strategies for Diabetes and their Limitations
| Strategy | Examples | Benefits | Limitations |
| Lifestyle Modifications | Healthy diet, regular physical activity | Improves blood glucose control and overall health | Difficult to maintain due to dietary preferences, physical limitations, socioeconomic constraints |
| Insulin Therapy | Exogenous insulin (T1DM, advanced T2DM) | Essential for T1DM, effective in controlling hyperglycemia | Risk of hypoglycemia, weight gain, lifelong administration required |
| Oral Hypoglycemic Agents | Metformin, Sulfonylureas, DPP-4 inhibitors, SGLT2 inhibitors | Effective in T2DM management | Side effects: gastrointestinal issues, hypoglycemia, potential cardiovascular risks |
| GLP-1 Receptor Agonists | Exenatide, Liraglutide, Semaglutide | Enhance insulin secretion, cardiovascular benefits | Nausea, high cost |
Mechanisms of Action of Naringenin in Diabetes Management
Naringenin exerts multiple protective effects in diabetes through antioxidant, anti-inflammatory, insulin-sensitizing, and metabolic regulatory pathways. Its antioxidant activity involves scavenging reactive oxygen species (ROS) and enhancing the function of key enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). This reduces oxidative stress and lipid peroxidation markers like malondialdehyde (MDA), thereby protecting tissues from diabetes-related damage.34 Anti-inflammatory effects are mediated by inhibition of the nuclear factor-kappa B (NF-κB) pathway, which downregulates pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β. Naringenin also reduces the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), further dampening inflammation and improving insulin sensitivity in diabetic models.35 In addition, naringenin enhances insulin sensitivity by stimulating the IRS/PI3K/Akt signaling pathway and promoting translocation of glucose transporter type 4 (GLUT4) in muscle and adipose tissues, which facilitates glucose uptake and lowers blood glucose levels.36 Naringenin also modulates lipid metabolism by regulating enzymes involved in lipid processing, lowering lipid peroxidation, and improving lipid profiles, which helps reduce cardiovascular risk associated with diabetes. Experimental studies have demonstrated significant improvements in lipid parameters following supplementation.37 Collectively, these mechanisms contribute to improved glucose homeostasis, with naringenin supporting antioxidant defense, reducing inflammation, enhancing insulin action, and correcting dyslipidemia. This multifaceted activity highlights its therapeutic potential in managing diabetes and preventing its complications.
Pharmacokinetics and Bioavailability of Naringenin
The pharmacokinetics of naringenin are influenced by its poor water solubility, rapid metabolism, and limited systemic availability, which collectively restrict its therapeutic potential. Understanding its ADME, along with the factors affecting bioavailability and strategies to overcome these limitations, is essential for optimizing its clinical application.
ADME of Naringenin
Naringenin has poor water solubility, which limits its gastrointestinal absorption, although it is taken up mainly in the small intestine.38 After absorption, it binds to plasma proteins and distributes to lipid-rich organs such as the liver, kidneys, and adipose tissue. In the liver, it undergoes extensive phase II metabolism, predominantly glucuronidation and sulfation, while cytochrome P450 enzymes (CYP1A2, CYP3A4) contribute to phase I reactions.39 The resulting metabolites are eliminated largely through urine, with some biliary excretion, and the compound shows a relatively short half-life of a few hours to about a day depending on dose and route.40
Factors Affecting Bioavailability
Naringenin’s bioavailability is influenced by multiple factors (Fig. 2). Its poor water solubility limits gastrointestinal absorption, while extensive first-pass metabolism rapidly converts it into glucuronide and sulfate metabolites, reducing systemic availability40. Food components also modulate absorption, with fats enhancing solubilization and uptake, whereas fibers and phytates may hinder it.41 Formulation strategies, including liposomes, nanoparticles, and micelles, improve solubility, stability, and absorption. Moreover, individual variability, such as genetic differences in metabolic enzymes and gut microbiota composition, further impacts its pharmacokinetics and therapeutic effectiveness.
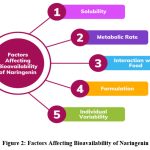 |
Figure 2: Factors Affecting Bioavailability of Naringenin |
Strategies to Improve Bioavailability
Several approaches have been developed to enhance the bioavailability of naringenin. Nanoparticle- and liposome-based delivery systems improve its solubility, protect against gastric degradation, and enhance intestinal absorption, resulting in higher systemic levels.42 Co-administration with compounds such as piperine can inhibit cytochrome P450 enzymes (CYP3A4, CYP1A2), reducing first-pass metabolism and increasing plasma concentrations.43 Prodrug strategies have also shown promise, improving solubility and permeability, with derivatives converted back to active naringenin post-absorption.44 Additionally, solubilizing agents like cyclodextrins and surfactants enhance dissolution in the gastrointestinal tract, further improving absorption and bioavailability.45
Safety and Toxicity
Naringenin, a flavonoid abundantly found in citrus fruits, possesses a favorable toxicological profile, making it generally safe for consumption. Extensive research has explored its safety aspects, revealing low acute toxicity and minimal adverse effects in animal models and human studies. While acute toxicity studies have demonstrated high LD50 values, indicating low toxicity levels, chronic toxicity investigations have not reported significant adverse effects associated with prolonged naringenin exposure.46-48 However, some studies have identified potential adverse effects, particularly at higher doses or in specific populations. These include gastrointestinal disturbances such as nausea and diarrhea, as well as potential drug interactions due to naringenin’s inhibition of certain cytochrome P450 enzymes involved in drug metabolism. Additionally, rare cases of allergic reactions have been reported. For long-term use, it is crucial to follow recommended dosage guidelines and monitor for adverse effects, particularly in individuals with existing health conditions or those taking medications. Although further research is required to completely understand the safety profile of naringenin, current evidence indicates that it can be safely included in a balanced diet, provided that dosage and individual factors are carefully considered.49,50
Potential for Therapeutic Use of Naringenin
Naringenin has emerged as a promising candidate for diabetes management, both as a standalone therapy and in combination with existing antidiabetic agents. Furthermore, innovative formulation strategies are being explored to overcome its limited bioavailability and optimize therapeutic efficacy. These aspects are summarized in Table 3.
Naringenin as a Standalone Therapy
Naringenin holds significant potential as a standalone therapy for managing diabetes and related metabolic disorders. As a natural flavonoid with antioxidant, anti-inflammatory, and insulin-sensitizing properties, naringenin offers multifaceted benefits for improving glucose metabolism, enhancing insulin sensitivity, and mitigating oxidative stress. Studies have demonstrated its ability to enhance pancreatic beta cell function, enhance glucose uptake in peripheral tissues and regulate key signaling pathways related to glucose homeostasis. By targeting multiple pathways implicated in diabetes pathogenesis, naringenin has the potential to exert comprehensive therapeutic benefits, positioning it as a promising option for standalone treatment.51
Synergistic Effects with Other Antidiabetic Agents
In addition to its efficacy as a standalone therapy, naringenin may exhibit synergistic effects when combined with other antidiabetic agents. Combinatorial therapy involving naringenin and conventional antidiabetic medications, such as metformin, sulfonylureas, or insulin, could offer enhanced efficacy and improved management of diabetes. Naringenin’s ability to modulate insulin signaling pathways, reduce inflammation, and improve lipid metabolism may complement the actions of existing antidiabetic drugs, leading to synergistic effects on glycemic control and metabolic parameters. Moreover, combining naringenin with other natural compounds with complementary mechanisms of action, such as resveratrol or berberine, could further potentiate its therapeutic effects, offering novel treatment strategies for diabetes management.52,53
Formulation Considerations
Formulation considerations play an essential role in optimizing the therapeutic potential of naringenin. Different formulation approaches, including nanoparticles, liposomes, and encapsulation, can improve the bioavailability, stability, and targeted delivery of naringenin, enhancing its therapeutic efficacy. Nanoparticle-based delivery systems allow for controlled release and sustained delivery of naringenin, prolonging its pharmacological effects and reducing dosing frequency. Encapsulation techniques safeguard naringenin from degradation in the gastrointestinal tract, enhancing its absorption and bioavailability. Furthermore, incorporating naringenin into functional foods or nutraceutical formulations could provide convenient and palatable options for long-term supplementation, promoting adherence to therapy and facilitating integration into dietary habits. By carefully considering formulation strategies, researchers can harness the full therapeutic potential of naringenin for effective management of diabetes and metabolic disorders.54,55
Table 3: Potential Therapeutic Applications of Naringenin in Diabetes Management
| Aspect | Key Features | Mechanism/Benefits |
| Naringenin as a Standalone Therapy | Natural flavonoid with antioxidant, anti-inflammatory, and insulin-sensitizing properties | Enhances pancreatic β-cell function, improves glucose uptake in peripheral tissues, regulates signaling pathways for glucose homeostasis, mitigates oxidative stress |
| Synergistic Effects with Other Antidiabetic Agents | Can be combined with metformin, sulfonylureas, insulin, or natural compounds (e.g., resveratrol, berberine) | Complements insulin signaling modulation, reduces inflammation, improves lipid metabolism; offers enhanced efficacy and better glycemic control |
| Formulation Considerations | Advanced drug delivery systems: nanoparticles, liposomes, encapsulation, functional foods | Improves bioavailability, stability, and targeted delivery; prolongs pharmacological effects; reduces dosing frequency; enhances absorption; enables integration into diet |
Challenges and Future Directions in Naringenin Research
Despite its therapeutic promise, naringenin research faces challenges such as poor bioavailability, limited clinical evidence, and lack of standardization. Addressing these gaps through mechanistic studies, safety evaluation, and advanced formulations will be crucial for its clinical translation.
Current Challenges in Naringenin Research
Key challenges in naringenin research include optimizing its bioavailability, as poor solubility and extensive first-pass metabolism limit systemic exposure, making advanced delivery systems like nanoparticles and encapsulation essential.56 Clinical translation also remains limited, with few well-designed trials and inconsistent findings slowing validation of efficacy and safety in humans. Moreover, lack of standardization in extraction, formulation, and quality control leads to variability in pharmacological outcomes, underscoring the need for standardized protocols to ensure reproducibility and reliability.57
Gaps in Knowledge and Research Needs
Despite growing evidence of naringenin’s pharmacological benefits, important research gaps remain. A clearer mechanistic understanding is needed, particularly in vivo, to identify the molecular pathways and cellular targets involved in its therapeutic effects in diabetes and metabolic disorders. Its long-term safety profile also requires further evaluation, including studies on potential toxicity, drug interactions, and risks at higher doses. Most critically, the lack of robust clinical evidence remains a major barrier; well-designed, large-scale randomized controlled trials with extended follow-up are essential to validate its efficacy and safety in humans.58
Potential Future Research Directions
Future research on naringenin should prioritize well-designed clinical trials to establish its safety, efficacy, and long-term benefits in diabetes and metabolic disorders, including comparative studies with standard therapies and potential synergistic effects with other antidiabetic agents. Mechanistic investigations in animal and cellular models are needed to clarify its interactions with key pathways regulating glucose metabolism, inflammation, and oxidative stress. In parallel, optimization of formulations through advanced delivery systems, such as nanotechnology-based approaches, offers a promising strategy to overcome bioavailability challenges.59,60 Addressing current limitations through collaborative efforts between researchers, clinicians, and industry will be essential to fully realize naringenin’s therapeutic potential and translate it into clinical practice.
Conclusion
In this review, we explored the potential of naringenin as a therapeutic agent for diabetes management. Naringenin, a flavonoid abundantly found in citrus fruits, exhibits antioxidant, anti-inflammatory, insulin-sensitizing, and glucose-regulating properties. By modulating key cellular pathways involved in glucose metabolism, insulin signaling, inflammation, and oxidative stress, it shows promise in improving glycemic control and overall metabolic health. Naringenin therefore represents a valuable candidate for developing innovative strategies and adjunctive therapies in diabetes, offering potential benefits in reducing complications and enhancing quality of life.
Acknowledgement
We thank management of KBHSS Trust’s Institute of Pharmacy, Malegaon for their continuous support and encouragement.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of IInterest
The authors do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Permission to reproduce material from other sources
Not Applicable
Author contributions
Kajal Pansare prepared the manuscript.
Yogesh Ahire assisted in writing and literature support.
Vinod Bairagi supervised the work and reviewed the manuscript.
References
- American Diabetes Association. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S14-S31. DOI: 10.2337/dc20-S002
CrossRef - World Health Organization. Global report on diabetes. Geneva, Switzerland: World Health Organization; 2024.
- Yen FS, Wei JC, Shih YH, Hsu CC, Hwu CM. Impact of individual microvascular disease on the risks of macrovascular complications in type 2 diabetes: a nationwide population-based cohort study. Cardiovascular diabetology. 2023 May 9;22(1):109. DOI: 10.1186/s12933-023-01821-8
CrossRef - Kumar A, Gangwar R, Ahmad Zargar A, Kumar R, Sharma A. Prevalence of diabetes in India: A review of IDF diabetes atlas 10th edition. Current diabetes reviews. 2024 Jan 1;20(1):105-14. DOI: 10.2174/1573399819666230413094200
CrossRef - Li R, Shen M, Yang Q, Fairley CK, Chai Z, McIntyre R, Ong JJ, Liu H, Lu P, Hu W, Zou Z. Global diabetes prevalence in COVID-19 patients and contribution to COVID-19–related severity and mortality: a systematic review and meta-analysis. Diabetes Care. 2023 Apr 1;46(4):890-7. DOI: 10.2337/dc22-1943
CrossRef - Weinberg Sibony R, Segev O, Dor S, Raz I. Drug therapies for diabetes. International Journal of Molecular Sciences. 2023 Dec 5;24(24):17147. DOI: 10.3390/ijms242417147
CrossRef - Larsen AH, Wiggers H, Dollerup OL, Jespersen NR, Hansson NH, Frøkiær J, Brøsen K, Nørrelund H, Bøtker HE, Møller N, Jessen N. Metformin lowers body weight but fails to increase insulin sensitivity in chronic heart failure patients without diabetes: a randomized, double-blind, placebo-controlled study. Cardiovascular Drugs and Therapy. 2021 Jun;35(3):491-503. DOI: 10.1007/s10557-020-07050-5
CrossRef - Liu YH, Liu Y, Zhang X, Fang L, Zhao BL, Wang NP. Activation of the endocannabinoid system mediates cardiac hypertrophy induced by rosiglitazone. Acta Pharmacologica Sinica. 2022 Sep;43(9):2302-12. DOI: 10.1038/s41401-022-00858-x
CrossRef - Datta S, Bhattacharjee S, Seal T. Anti-diabetic, anti-inflammatory and anti-oxidant properties of four underutilized ethnomedicinal plants of West Bengal, India: an in vitro approach. South African Journal of Botany. 2022 Sep 1;149:768-80. DOI: 10.1016/j.sajb.2022.06.029
CrossRef - Di Giacomo C, Malfa GA, Tomasello B, Bianchi S, Acquaviva R. Natural compounds and glutathione: Beyond mere antioxidants. Antioxidants. 2023 Jul 18;12(7):1445. DOI: 10.3390/antiox12071445
CrossRef - Atrahimovich D, Avni D, Khatib S. Flavonoids-macromolecules interactions in human diseases with focus on Alzheimer, atherosclerosis and cancer. Antioxidants. 2021 Mar 10;10(3):423. DOI: 10.3390/antiox10030423
CrossRef - Cahyana Y, Adiyanti T. Flavonoids as antidiabetic agents. Indonesian Journal of Chemistry. 2021 Feb 2;21(2):512-26. DOI: 10.22146/ijc.58439
CrossRef - De Rossi L, Rocchetti G, Lucini L, Rebecchi A. Antimicrobial potential of polyphenols: Mechanisms of action and microbial responses—A narrative review. 2025;14(2):200. DOI: 10.3390/antiox14020200
CrossRef - Stiller A, Garrison K, Gurdyumov K, et al. From fighting critters to saving lives: polyphenols in plant defense and human health. International journal of molecular sciences. 2021 Aug 20;22(16):8995. DOI: 10.3390/ijms22168995
CrossRef - Dzah CS, Asante-Donyinah D, Letsyo E, Dzikunoo J, Adams ZS. Dietary polyphenols and obesity: A review of polyphenol effects on lipid and glucose metabolism, mitochondrial homeostasis, and starch digestibility and absorption. Plant Foods for Human Nutrition. 2023 Mar;78(1):1-2. DOI: 10.1007/s11130-022-01034-6
CrossRef - Drețcanu G, Știrbu I, Leoplold N, et al. Chemical structure, sources and role of bioactive flavonoids in cancer prevention: a review. Plants. 2022 Apr 20;11(9):1117. DOI: 10.3390/plants11091117
CrossRef - Kumar H, Guleria S, Kimta N, et al. Applications of citrus peels valorisation in circular bioeconomy. J Agric Food Res. 2025;20:101780. DOI: 10.1016/j.jafr.2025.101780
CrossRef - Ebrahimi F, Ghazimoradi MM, Fatima G, Bahramsoltani R. Citrus flavonoids and adhesion molecules: potential role in the management of atherosclerosis. Heliyon. 2023 Nov 1;9(11). DOI: 10.1016/j.heliyon.2023.e21849
CrossRef - Shin JH, Shin SH. A comprehensive review of naringenin, a promising phytochemical with therapeutic potential. J Microbiol Biotechnol. 2024;34(12):2425-2438. DOI: 10.4014/jmb.2410.10006
CrossRef - PubChem [online]. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Naringenin
- Avila EP, Mendes LA, De Almeida WB, Dos Santos HF, De Almeida MV. Conformational analysis and reactivity of naringenin. Journal of Molecular Structure. 2021 Dec 5;1245:131027. DOI: 10.1016/j.molstruc.2021.131027
CrossRef - Halevas E, Mavroidi B, Zahariou G, Pelecanou M, Hatzidimitriou AG. Structurally characterized copper complexes of flavonoid naringenin with enhanced radical scavenging activity. Inorganica Chimica Acta. 2023 Feb 1;546:121325. DOI: 10.1016/j.ica.2022.121325
CrossRef - Alimohammadi M, Mohammad RN, Rahimi A, Faramarzi F, Alizadeh-Navaei R, Rafiei A. The effect of immunomodulatory properties of naringenin on the inhibition of inflammation and oxidative stress in autoimmune disease models: a systematic review and meta-analysis of preclinical evidence. Inflammation Research. 2022 Nov;71(10):1127-42. DOI: 10.1007/s00011-022-01599-7
CrossRef - Singh S, Sharma A, Monga V, Bhatia R. Compendium of naringenin: potential sources, analytical aspects, chemistry, nutraceutical potentials and pharmacological profile. Crit Rev Food Sci Nutr. 2023;63(27):8868-8899. DOI: 10.1080/10408398.2022.2056726
CrossRef - Sims EK, Besser RE, Dayan C, Geno Rasmussen C, Greenbaum C, Griffin KJ, Hagopian W, Knip M, Long AE, Martin F, Mathieu C. Screening for type 1 diabetes in the general population: a status report and perspective. Diabetes. 2022 Apr 1;71(4):610-23. DOI: 10.2337/dbi20-0054
CrossRef - American Diabetes Association. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S14-S31.
CrossRef - You H, Hu J, Liu Y, Luo B, Lei A. Risk of type 2 diabetes mellitus after gestational diabetes mellitus: A systematic review & meta-analysis. Indian Journal of Medical Research. 2021 Jul 1;154(1):62-77. DOI: 10.4103/ijmr.IJMR_852_18
CrossRef - Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. The Lancet. 2022 Nov 19;400(10365):1803-20. DOI: 10.1016/S0140-6736(22)01655-5
CrossRef - Sharma AK, Singh S, Singh H, Mahajan D, Kolli P, Mandadapu G, Kumar B, Kumar D, Kumar S, Jena MK. Deep insight of the pathophysiology of gestational diabetes mellitus. 2022 Aug 28;11(17):2672. DOI: 10.3390/cells11172672
CrossRef - Thethi TK, Bilal A, Pratley RE. Cardiovascular outcome trials with glucose-lowering drugs. Current Cardiology Reports. 2021 Jul;23(7):75. DOI: 10.1007/s11886-021-01505-3
CrossRef - Carmichael J, Fadavi H, Ishibashi F, Shore AC, Tavakoli M. Advances in screening, early diagnosis and accurate staging of diabetic neuropathy. Frontiers in endocrinology. 2021 May 26;12:671257. DOI: 10.3389/fendo.2021.671257
CrossRef - Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nature reviews cardiology. 2023 Jul;20(7):463-74. DOI: 10.1038/s41569-023-00849-3
CrossRef - Mansour A, Mousa M, Abdelmannan D, Tay G, Hassoun A, Alsafar H. Microvascular and macrovascular complications of type 2 diabetes mellitus: Exome wide association analyses. Frontiers in Endocrinology. 2023 Mar 23;14:1143067. DOI: 10.3389/fendo.2023.1143067
CrossRef - Li SH, Wang MS, Ke WL, Wang MR. Naringenin alleviates myocardial ischemia reperfusion injury by enhancing the myocardial miR-126-PI3K/AKT axis in streptozotocin-induced diabetic rats. Experimental and Therapeutic Medicine. 2021 Aug;22(2):810. DOI: 10.3892/etm.2021.10242
CrossRef - Stabrauskiene J, Kopustinskiene DM, Lazauskas R, Bernatoniene J. Naringin and naringenin: Their mechanisms of action and the potential anticancer activities. 2022;10(7):1686. DOI: 10.3390/biomedicines10071686
CrossRef - Huang M, Deng M, Nie W, Zou D, Wu H, Xu D. Naringenin inhibits platelet activation and arterial thrombosis through inhibition of phosphoinositide 3-kinase and cyclic nucleotide signaling. Frontiers in Pharmacology. 2021 Aug 12;12:722257. DOI: 10.3389/fphar.2021.722257
CrossRef - Ahmad A, Prakash R, Khan MS, Altwaijry N, Asghar MN, Raza SS, Khan R. Enhanced antioxidant effects of naringenin nanoparticles synthesized using the high-energy ball milling method. ACS omega. 2022 Sep 19;7(38):34476-84. DOI: 10.1021/acsomega.2c04148
CrossRef - Hu L, Luo Y, Yang J, Cheng C. Botanical flavonoids: Efficacy, absorption, metabolism and advanced pharmaceutical technology for improving bioavailability. Molecules. 2025;30(5):1184. DOI: 10.3390/molecules30051184
CrossRef - Di Lorenzo C, Colombo F, Biella S, Stockley C, Restani P. Polyphenols and human health: The role of bioavailability. Nutrients. 2021 Jan 19;13(1):273. DOI: 10.3390/nu13010273
CrossRef - Barrera-Reyes PK, Cortés-Fernández de Lara J, Poquet L, Redeuil K, Kussmann M, Silva-Zolezzi I, Tejero EM. Circulating structurally related (-)-epicatechin metabolite species and levels after sustained intake of a cocoa powder high in polyphenols are comparable to those achieved after a single dose. Nutrients. 2021 Oct 27;13(11):3829. DOI: 10.3390/nu13113829
CrossRef - Madkour DA, Ahmed M, Elkirdasy AF, Orabi SH, Mousa AA. Rutin: Chemical properties, Pharmacokinetic properties and Biological activities. Matrouh Journal of Veterinary Medicine. 2024 Nov 1;4(1):26-34. DOI: 10.21608/mjvm.2024.341806
CrossRef - Farhan M, Rizvi A, Aatif M, Ahmad A. Current understanding of flavonoids in cancer therapy and prevention. Metabolites. 2023;13(4):481. DOI: 10.3390/metabo13040481
CrossRef - Patil P, Gupta M, Semwal A, Tote MV, Divakar MC, Dodiya R. Reviewing Bioenhancers in Traditional Indian Medicine: Exploring their Potential Mechanisms and Applications. Frontiers in Health Informatics. 2024 Dec 1;13(8).
- Al-Ishaq RK, Liskova A, Kubatka P, Büsselberg D. Enzymatic metabolism of flavonoids by gut microbiota and its impact on gastrointestinal cancer. Cancers. 2021 Aug 4;13(16):3934. DOI: 10.1155/2023/4839124
CrossRef - Gera S, Sampathi S, Maddukuri S, Dodoala S, Junnuthula V, Dyawanapelly S. Therapeutic potential of naringenin nanosuspension: in vitro and in vivo anti-osteoporotic studies. 2022 Jul 11;14(7):1449. DOI: 10.3390/pharmaceutics14071449
CrossRef - Dias MC, Pinto DC, Silva AM. Plant flavonoids: Chemical characteristics and biological activity. Molecules. 2021 Sep 4;26(17):5377. DOI: 10.3390/molecules26175377
CrossRef - Alam F, Mohammadin K, Shafique Z, Amjad ST, Asad MH. Citrus flavonoids as potential therapeutic agents: A review. Phytotherapy Research. 2022 Apr;36(4):1417-41. DOI: 10.1002/ptr.7261
CrossRef - Jiang YP, Wang S, Lai WD, et al. Neuronal CRMP2 phosphorylation inhibition by the flavonoid, naringenin, contributes to the reversal of spinal sensitization and arthritic pain improvement. Arthritis research & therapy. 2022 Dec 23;24(1):277. DOI: 10.1186/s13075-022-02975-8
CrossRef - Mistry J, Biswas M, Sarkar S, Ghosh S. Antidiabetic activity of mango peel extract and mangiferin in alloxan-induced diabetic rats. Future Journal of Pharmaceutical Sciences. 2023 Mar 20;9(1):22. DOI: 10.1186/s43094-023-00472-6
CrossRef - Kumavath R, Paul S, Pavithran H, Paul MK, Ghosh P, Barh D, Azevedo V. Emergence of cardiac glycosides as potential drugs: Current and future scope for cancer therapeutics. Biomolecules. 2021 Aug 25;11(9):1275. DOI: 10.3390/biom11091275
CrossRef - Adefegha SA, Dada FA, Oyeleye SI, Oboh G. Effects of berberine on cholinesterases and monoamine oxidase activities, and antioxidant status in the brain of streptozotocin (STZ)-induced diabetic rats. Journal of Basic and Clinical Physiology and Pharmacology. 2022 Aug 9;33(4):389-97. DOI: 10.1515/jbcpp-2020-0173
CrossRef - Bateni Z, Behrouz V, Rahimi HR, Hedayati M, Afsharian S, Sohrab G. Effects of nano-curcumin supplementation on oxidative stress, systemic inflammation, adiponectin, and NF-κB in patients with metabolic syndrome: A randomized, double-blind clinical trial. Journal of Herbal Medicine. 2022 Feb 1;31:100531. DOI: 10.1016/j.hermed.2021.100531
CrossRef - Kim J. Pre-clinical neuroprotective evidences and plausible mechanisms of Sulforaphane in Alzheimer’s disease. International journal of molecular sciences. 2021 Mar 13;22(6):2929. DOI: 10.3390/ijms22062929
CrossRef - Li R, Peng Y, Pu Y, Zhao Y, Nie R, Guo L, Wu Y. Fructose and biotin co-modified liposomes for dual-targeting breast cancer. Journal of Liposome Research. 2022 Apr 3;32(2):119-28. DOI: 10.1080/08982104.2021.1894171
CrossRef - Fatima N, Khan MI, Jawed H, Qureshi U, Ul-Haq Z, Hafizur RM, Shah TA, Dauelbait M, Bin Jardan YA, Shazly GA. Cinnamaldehyde ameliorates diabetes-induced biochemical impairments and AGEs macromolecules in a pre-clinical model of diabetic nephropathy. BMC Pharmacology and Toxicology. 2024 Nov 14;25(1):85. DOI: 10.1186/s40360-024-00811-0
CrossRef - Ke L. Naringenin as a neurotherapeutic agent in Alzheimer’s disease: Epigenetic signatures, gut microbiota alterations, and molecular neuroprotection. Frontiers in Aging Neuroscience. 2025 Aug 15;17:1647967. DOI: 10.3389/fnagi.2025.1647967
CrossRef - Kaźmierczak T, Cyboran-Mikołajczyk S, Trochanowska-Pauk N, Walski T, Nowicka P, Bonarska-Kujawa D. Insights on the Mechanisms of the Protective Action of Naringenin, Naringin and Naringin Dihydrochalcone on Blood Cells in Terms of Their Potential Anti-Atherosclerotic Activity. Molecules. 2025 Jan 25;30(3):547. DOI: 10.3390/molecules30030547
CrossRef - Barve RV, Kotade KB, Bhandare SN, Gaikar MT, Dhamak VM. Enhancing analgesic and anti-inflammatory synergetic effect with Naringenin: Insilico and in vivo Tropical Journal of Natural Product Research. 2025 May 1;9(5), p2141. DOI: 10.26538/tjnpr/v9i5.38
CrossRef - Veiko AG, Lapshina EA, Zavodnik IB. Comparative analysis of molecular properties and reactions with oxidants for quercetin, catechin, and naringenin. Molecular and Cellular Biochemistry. 2021 Dec;476(12):4287-99. DOI: 10.1007/s11010-021-04243-w
CrossRef - Yajima S, Sakata R, Watanuki Y, Sashide Y, Takeda M. Naringenin Suppresses the Hyperexcitability of Trigeminal Nociceptive Neurons Associated with Inflammatory Hyperalgesia: Replacement of NSAIDs with Phytochemicals. Nutrients. 2024 Nov 15;16(22):3895. DOI: 10.3390/nu16223895
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





