Manuscript accepted on : 11-08-2025
Published online on: 10-09-2025
Plagiarism Check: Yes
Reviewed by: Dr. Seyedeh Maryam Mousavi
Second Review by: Dr. Deepali Bansode
Final Approval by: Dr. Wagih Ghannam
Formulation Strategies for Controlled Drug Release Using MUPS Tablets: Advances and Challenges
Sahebrao Sampat Boraste* and Deelip Vishram Derle
and Deelip Vishram Derle
Department of Pharmaceutics, MVP’S College of Pharmacy Nashik (Affiliated to Savitribai Phule Pune University), Pune, India
Corresponding Author Email: saheb2410@gmail.com
ABSTRACT: The multi-unit pellet system tablets, employing coated pellets for regulated drug release, functions as an efficacious therapeutic substitute for conventional immediate-release dosage forms. The principal advantages of multi-unit pellet system tablets are a) the convenience of administration and b) the capacity to be partitioned without compromising the drug release characteristics of the individual units. Multi-Unit Pellet System tablets can be manufactured more economically than pellet-filled capsules owing to the substantially greater production rate of the tableting process. Studying the literature one can find that despite the advantages of multi-unit pellet system tablets, its implementation has been hindered by manufacturing issues, including impaired integrity of coated pellets and inadequate content consistency. This paper presents a revised review of research concerning the compaction of multi-unit pellet system tablets, derived from both scholarly literature and patents. Essential elements for the effective development of these tablets are outlined, including model drug attributes, potential cushioning agents, and novel techniques to protect pellets from degradation. It aims to facilitate the future advancement of manufacturable multi-unit pellet system tablets that demonstrate drug release properties similar to those of the original coated pellets.
KEYWORDS: Coating of pellets; Cushioning agents; Multi-unit pellet system (MUPS); Tablet excipients; Tableting of pellets
| Copy the following to cite this article: Boraste S. S, Derle D. V. Formulation Strategies for Controlled Drug Release Using MUPS Tablets: Advances and Challenges. Biotech Res Asia 2025;22(3). |
| Copy the following to cite this URL: Boraste S. S, Derle D. V. Formulation Strategies for Controlled Drug Release Using MUPS Tablets: Advances and Challenges. Biotech Res Asia 2025;22(3). Available from: https://bit.ly/4phAvKq |
Introduction
When the route is Oral, Solid dose forms serve as the principal means of medicine delivery. They significantly vary in different parameters of units per dose, encompassing both single-unit dosage forms (SUDF) as well as multiple-unit dosage forms (MUPS).1 Unlike SUDF, the primary attribute of MUPSs is their minimal chance of the dumping of dose as well as rapid gastric emptying, as they can be more uniformly dispersed in the gastrointestinal region.2 This leads to diminished side effects, enhanced bioavailability, reduced variability in medication release, and other notable characteristics. They provide the benefits of enhanced patient adherence due to the simplicity of administration and the ability to customize dose fractionation. Moreover, MUPS can enable the concurrent delivery of incompatible pharmaceuticals or a medication within a normally incompatible excipient matrix by physical segregation.3
The MUPS creates an elevated hindrance to obstruct the entry of generic medications, hence extending the profitable viability of a specific drug.4 The MUPS is preferred owing to its unique physical attributes, including spherical morphology, and its mechanical properties, such as elasticity, deformability, and tensile strength.5 MUPS are typically enclosed or compressed with additives to create a tablet dosage form. Nowadays, multi-unit pellet system tablets (TMUPS) have attracted increased attention owing to their supplementary advantages. They can be produced using drug pellets which are not coated or functionally coated pellets, enabling customized liberation of medications.6
Tablets are ideal over capsules due to their superior convenience for patients, lower manufacturing costs, and increased production rates.7,8 High-dose MUPSs are strongly avoided due to insufficient patient adherence to large capsules. Conversely, (TMUPS) can deliver a larger volume of drugs while keeping the capacity same of MUPSs because to their enhanced density.9 Furthermore, they offer a segmented dose while preserving the drug release characteristics of each unit. Owing to several constraints in TMUPS manufacturing, a restricted quantity of commercial pharmaceutical TMUPS products is now accessible in the market.10
TMUPS must ideally decompose swiftly in the GI system following the delivery of drug, yielding release characteristics similar to that of uncompressed MUPS. The primary obstacle in the progression of TMUPS is the degradation of the functional coating due to compression, resulting in a subsequent loss of modified-release, taste-covering attributes.11 When utilizing purpose-coated MUPS, it is crucial to safeguard the polymeric coating against rupture during tableting, as any fissures in the layer may adversely affect the drug release characteristics. The study emphasizes the critical management of pellet dimensions, coating composition, tableting additives, as well as compaction parameters to preserve the desired drug release characteristics of TMUPS pellets.12
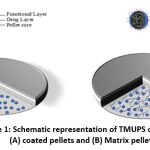 |
Figure 1: Schematic representation of TMUPS containing (A) coated pellets and (B) Matrix pellets. |
Pellet core
Composition of pellet core
The flexibility of pellet cores affects the tablet ability of pellets as well as the integrity of the purpose coating film during the tableting process. Stiffer pellet cores exhibit reduced deformation under compression, which is advantageous for preserving the integrity of the coating. The mechanical qualities of cores are contingent upon their compositions. Since the 1990s, MCC pellet cores are extensively utilized in TMUPS. The primary mechanism of permanent deformation in the MCC pellets is plastic deformation. The inclusion of more rigid excipients is anticipated to enhance the compression resistance of the pellet; nevertheless, it simultaneously increases the likelihood of core fractures during compression.13
The outcomes indicated that those pellets which had 80% dicalcium phosphate dihydrate & 20% MCC exhibited superior resistance to deformation compared to MCC pellets alone, with minimal fragmentation seen. Combinations of microcrystalline cellulose (MCC) and plastic excipients, such as MCC/polyethylene glycol (PEG), were utilized to produce pellet cores with enhanced deformability. Recently, pellets comprising cellulose derivatives have been increasingly employed to produce sustained-release tablets with elevated pharmaceutical content.14
MCC is not generally used when pharmaceuticals demonstrate chemical incompatibility with MCC or when drug release from the core is required, as MCC-based cores generally do not dissolve swiftly. In these cases, κ-carrageenan has been employed as a replacement for MCC, as its inclusion in the pellets promotes rapid core dissolution and hence expedited drug release. κ-Carrageenan pellets offer improved formulation stability, enabling the integration of fillers and active ingredient concentrations, making them ideal for enteric-coated pellets for rapid medication release in the intestine after gastric passage. Inert pellets made of isomalt and sugar have been employed to manufacture TMUPS. Nonetheless, their utilization in tableting has been constrained because to the considerable fracturing of the sugar cores, which presumably causes major damage to the coating layer during the tableting process.15,16
Pellet core porosity
This parameter of pellets affects their deformation and densification, thus affecting their compression and release properties. Research examining the results of pellet porosity with ethyl cellulose coated reservoir pellets demonstrated that the factor in question was substantially affected the level of densification, while exerting only a minimal influence on the release of the drug. Compaction had a much smaller impact on the deterioration of pellets with greater porosity.17 Hence, to achieve the best drug release qualities of TMUPS, this parameter must be carefully controlled. The porosity of pellets influences their deformation and densification, thus affecting their compression and release properties.18
Pellet core size
The dimensions of the core of the pellets have been proven to influence the compaction characteristics as well as the medication release from the fragmented pellets. Johansson and colleagues examined the influence of particle dimensions (which ranged from 425 to 500 µm as well as 1250 to 1400 µm) on the process of compression of pellets. The larger pellets exhibited greater deformation than those which were less in size.19 The pellets which were greater in size diminished the quantity of force transmission sites, resulting in heightened contact stress at each location and hence leading to higher deformation rates. Large-sized pellet cores led to increased damage to the layer as well as increased irregularities in the delivery properties of both types of pellets. Likewise, pellets which are small in size as well as coated endured reduced stress while compression operation due to their enhanced ability to fill the spaces within the excipient matrix.20 Nonetheless, as the weight of the coating material increased, smaller pellets demonstrated a reduced thickness of the enteric coating and a more rapid drug release from TMUPS. Because the effects of pellet deformation & coating thickness of layers vary with pellet size, it is necessary to empirically determine the ideal pellet size for a certain coating method.21
Coating
Common polymers used for the coating
Sustained release coating
Prevalent polymers employed for sustained release include polyvinyl acetate and ethyl cellulose, both of which possess FDA approval.22 The evaluation of the drug release rate prior to and subsequent to compression indicated that pellets coated with Kollicoat® SR 30D have the potential to be compressed into tablets without substantially affecting the integrity of the coating; conversely, Aquacoat® ECD 30 as well as Surelease® layer of coatings were affected during compression. The minimal elongation (less than 5 percent) as well as inadequate physical characteristics of EC in that investigation partially elucidated this phenomenon. Increasing the concentration of plasticizer in the coating material or providing a thicker coating layer could help address this issue.23
pH-related enteric/colonic coating
Resins of acrylic, branded as Eudragit® and Kollicoat®, comprise polymers that demonstrate varying pH conditional solubility within the range of 5.5 to 7.0 pH, suitable for enteric and colonic drug transport applications. It is possible to compress pellets covered with HPMC as well as Eudragit® S 100 in tablets without sacrificing the coat’s functional characteristics. The highly elongated Eudragit® FS 30D creates uniform films. The study looked at various combinations of Eudragit® FS 30D & Eudragit® L 30D-55 mixes. The study was able to obtain TMUPS by carefully choosing cushioning excipients and improving the coating formulation. For this experiment, a ratio of one to one of Eudragit® FS 30D and Eudragit® L 30D-55 was utilized, with the latter showing greater efficiency.24,25
Reverse enteric coating
Eudragit® EPO is miscible at the reading of pH below 5, whereas it becomes swellable and permeable at pH values over 5. This unique pH-dependent property restricts drug release in saliva while promoting breakdown in gastric fluids, making it ideal for coatings used to alter or mask taste. It is thus inappropriate for taste-altering acidic medications because of compatibility concerns [26,27]. Eudragit® RL 30D may effectively substitute Eudragit® EPO, as evidenced by the successful production of orally disintegrating tablets which are taste-masked and contain Cetirizine HCl. The coating or layering of Shellac was utilized to conceal the undesirable flavour of acetaminophen. Research on coating solutions for taste-masking with regards to TMUPS is limited, possibly due to the adverse mechanical features of these polymers, such as inadequate extensibility, which impede the maintenance of taste integrity. Masking films during compression.28
Coating level
The level of coating is meticulously connected to the thickness of the film and hence affects the profile of drug release.29 These patterns of drugs can be altered only by regulating the coating thickness. Augmenting coating thickness may enhance the layer’s resilience to harm from compression. The coating thickness of the pellets determines their endurance, which in turn delays the release of the medication.30 One solution to this problem is to utilize a pore-former, such as polysorbate 80 or HPMC. Protecting the functional coating layer and allowing direct tableting of TMUPS requiring cushioning additives are both achieved by using a protective polymer topcoat, such as polyvinyl pyrrolidone K30 or PVP VA64. While keeping the functional coating layer intact, the increased flexibility of the top-coated polymer layer enables extensive bonding contact between nearby pellets at moderate pressures. The tablet ability and drug release properties of the pellets with the top coating determine the optimal thickness.31
Plasticizers
To keep the releasing qualities of TMUPS and to account for pellet deformation while keeping the functional coating intact, the coating layers need to be extended by 75%.32 Compression can cause brittle coating films to break, thus plasticizers that are well-suited with polymers and may decrease their glass transition temperatures are added.33 Using Killicoat® SR 30D-coated verapamil hydrochloride floating pellets, the research examined the effects of three plasticizers: TEC, DBS, and PPG. Because the plasticizer affects the dispersion of medication via the Kollicoat® SR 30D coating layer, TEC and DBS exhibited delayed medication release in comparison to PPG. An efficient plasticizer was determined to be water. The study found that coated pellets cannot be compressed effectively without moisture, and the films coated with Eudragit® L showed brittleness in dry conditions. However, the compression-induced deterioration of the coating layer was reduced with increased humidity and storage period. This was concluded after storing the pellets at different ranges of humidities.34 Pellets loaded with Eudragit® L can be effectively compressed due to the plasticization of Eudragit® L films by moisture at elevated humidity, indicating that TEC as a plasticizer is not effective. Moisture influences the compressive properties of compacts and the porosity of tablets due to its plasticizing effect. Once the framework of the pore is established, the moisture/ water content seldom influences the kinetics of the release of the active agent. Disintegration and rapid release occurred at a value of porosity exceeding 0.075. Any value below this threshold, the tablets remained complete and demonstrated sustained-release properties notwithstanding crack formation.35
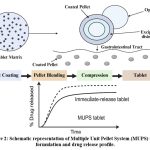 |
Figure 2: Schematic representation of Multiple Unit Pellet System (MUPS) tablet formulation and drug release profile. |
Tableting excipients
Types of excipients
Drug release during compaction is a problem for the production of TMUPS36. One way to address this is by using excipients as a buffering agent when taking pellets by tablet. To fill the empty area between the densely packed pellets, approximately 29% excipient is required. Because of its plastic deformability, MCC finds widespread application. Significant mechanical strength and low friability can be achieved with CeolusTM KG-801. The significant deformation of Ceolus™ KG-801 granules reduces the deformation of the coated pellets. The Ceolus™ UF-711 product from MCC has more sphericity compared to many other MCC particles, hence indicating an increased flowability. Its high porosity makes it highly collapsible. Ceolus™ UF-711 was utilized efficiently alongside a little quantity of Ceolus™ PH-200 to formulate TMUPS, demonstrating negligible blend isolation and enteric-release characteristics. Conventional disintegrants may affect the tableting efficacy of TMUPS. While PVP XL tablets exhibited less friability and increased hardness, they disintegrated faster than CMC-Na and CMS-Na tablets. Pellets coated with xanthan gum, budesonide-loaded, L30D-55, 25%, and Eudragit® NE are some examples. A full day later, 84% of the medicine had been released into the stomach and intestines thanks to the protected release of Eudragit® FS 30D. Cellactose® 80 granules were utilized to manufacture TMUPS that exhibited satisfactory mechanical qualities while maintaining medication release patterns.37,38
Ratio of pellets to additives/excipients
Tablet characteristics such as friability, disintegration duration are impacted by the pellet to excipients ratio, which in turn impacts the compacting procedure of pellets [39]. Sustained-release covered diltiazem pellets that contained wax/starch as a cushioning ingredient worked well. Using half waxy pellets produced a medication distribution pattern that was comparable to that of uncompressed granules. The dissolution velocity was enhanced due to the breakdown of the coating layer when the quantity of cushioning pellets was reduced to 40%. Because there was more hydrophobic wax, the dissolving rate of pills with 60% cushioning pellets was slower. Parameters like the mechanical strength, disintegration of Piroxicam tablets were impacted by the use of enteric-coated pellets in their formulation.40
Dimensions of cushioning excipients
Smaller particles serve as more effective cushioning agents, and the granulation of excipients that aligns with pellet size enhances the uniformity of medication content. Micronized lactose, compressed with enteric-coated pellets, provides enhanced adaptability for particle reconfiguration. This material is employed as a cushioning agent. It relieves compression tension by adhering to coated pellet surfaces, which is achieved through a lubricating effect. The enhanced protection against compression damage is provided by smaller lactose particles as a result of the increased contact points. A study conducted on the production of TMUPS with MCC granules as a buffering excipient discovered that the segregation of the pellet-excipient blend during tableting was reduced by larger particles. Consequently, it is imperative to empirically determine the optimal dimensions of the cushioning excipients during the formulation of TMUPS products.41
Equipment
Compaction pressure
Tablet characteristics and the distribution of drug release in compacted pellets such as diltiazem as well as ibuprofen are impacted by compression pressure. The strength, disintegration duration, and friability of tablets are greatly reduced, but the drug release is unaffected. Friability of TMUPS decreases as compaction force increases. More robust tablets are manufactured at increased pressures within the conventional compaction range of 100 – 300 MPa, provided that over-compression is circumvented. In viscoelastic materials that endure significant time-dependent deformation under pressure, a reduced compression force coupled with an extended dwell period can improve tablet strength and reduce friability. These materials demonstrate elastic properties at high tableting speeds, resulting in the inability to form durable tablets even under considerable pressure. However, extended exposure to stress may lead to greater plastic deformation than under increased pressure, albeit at a faster rate.42
Tablet shape
The tooling architecture significantly impacts stress distribution within tablets and affects tableting additive distortion. A polymer (5% TEC) and antiadherent (32.2% talc) agent were used to cover drug-loaded pellets with Eudragit® FS 30D. Biconvex tablet tooling caused less damage to enteric-coated pellets and increased tablet mass from 430 to 970 mg. This reduced contact between pellet and punch, decreased friability to 0.1%, and increased hardness to 85 N.43
Innovative compaction approaches and rising cushioning compounds
Emerging cushioning agents
Novel TMUPS cushioning compounds are being looked for to safeguard functioning coatings and forestall the formation of non-disintegrating particles. The significant amount of plastically flexible wax in composites that utilize glyceryl monostearate (GMS) makes them attractive. Their effectiveness in preventing the functional coating of pellets from degradation under different tableting techniques has been demonstrated. Compression can do less harm when using soft paraffinic wax beads. The padding is best achieved by co-processing spray-dried micronized lactose with polymers such as HPC.44
Novel compaction approaches:
Application of tableting excipients via spray-layering onto pellets
One way to improve flow properties and solve segregation issues is to spray-layer tableting excipients onto pellets. A novel mesalamine TMUPS was developed using this technology. It dissolves in the upper part of the gastrointestinal system and releases pellets having the drug in the colon. Additionally, a glipizide TMUPS was produced using this approach; it exhibited sustained release properties with kinetics close to zero-order and delayed drug release by two hours. New drug delivery methods were developed using this technology.45
Hot tableting
This can generate TMUPS from coated pellets using much reduced compression forces compared to conventional tableting methods. The minimum compression force enables the production of TMUPS from pressure-sensitive components, such as slow-release pellets and enzymes. The hot tableting method was used to produce tablets with EC-coated tramadol hydrochloride pellets, ensuring the tablets’ integrity and optimal physical characteristics.46
Compaction with pellet-containing granules
A uniform mixture of coated pellets as well as cushioning granules for premium tablets can be made using pellet-containing granules. Tablets will be durable, the coating layer will be protected, and segregation problems will be reduced using this procedure. When contrasted with tablets manufactured from coated pellets and excipients, those with pellet-containing granules exhibit superior weight and drug content consistency.47
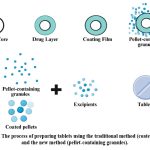 |
Figure 3: The process of preparing tablets using the traditional method (coated pellets) and the new method (pellet-containing granules). |
Recent patents
WO2012017074 and WO2013092497 created esomeprazole and TMUPS oral formulations with minimum compression force, respectively. They obtained the desired hardness and friability in their tablets by compressing pellets that were enterically coated. WO2013092497 resolved problems with non-disintegrating aggregates and release-controlling coatings by introducing an oral immediate-release tablet of dabigatran etexilate. A new daily dosage unit including three or more antiviral medications was created using PULSYS™ technology to enhance antibiotic treatment methods. The trifecta of an immediate-release, a delayed-release, and a delayed-release component makes up a 775 mg amoxicillin tablet with pulses.
Conclusion
TMUPS, a medication delivery system, is being investigated for its potential advantages, including controlled drug release, enhanced production efficiency, and increased patient convenience. The creation of TMUPS is a problem because to the necessity of meticulously assessing the pellet core, polymeric covering, and cushioning excipients. The core must possess hardness and pliability, whilst the coating must exhibit strength, flexibility, and thickness. The cushioning excipients must closely mimic the coated pellets to avert segregation. The proportion of pellets to tableting excipients must be meticulously selected to maintain the integrity of the functional coating layer and the uniformity of medication content. Innovations in materials and techniques are anticipated to enhance the prominence of TMUPS in future pharmaceutical delivery. The obstacles related to MUPS tablet compression, including the attainment of consistent pellet size distribution, the resolution of coating complications, and the consideration of tooling, highlight the necessity for a thorough and cohesive strategy. Using advanced strategies & technologies, precisely monitoring parameters and controls as well as meticulous quality control becomes paramount for the utility and progression of these dosage forms in the future.
Acknowledgment
The authors are thankful to MVP’s College of Pharmacy Nashik and Savitribai Phule Pune University, Pune.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required. Clinical Trial.
Clinical Trial Registration
This research does not involve any clinical trials.
Permission to reproduce material from other sources
Not Applicable.
Author Contributions:
Sahebrao Boraste: Conceptualization, Visualization, Methodology, Data Collection, Writing.
Deelip Derle: Visualization, Supervision, Review & Editing.
References:
- Murugesan S, Gowramma B, Lakshmanan K, Reddy Karri VV, Radhakrishnan A. Oral modified drug release solid dosage form with special reference to design; an overview. Current Drug Research Reviews Formerly: Current Drug Abuse Reviews. 2020; 12(1):16-25. DOI: https://doi.org/10.2174/2589977511666191121094520.
CrossRef - Newton JM. Gastric emptying of multi-particulate dosage forms. International journal of pharmaceutics. 2010; 395(1-2):2-8. DOI: https://doi.org/10.1016/j.ijpharm.2010.04.047.
CrossRef - Aremu TO, Oluwole OE, Adeyinka KO, Schommer JC. Medication adherence and compliance: recipe for improving patient outcomes. Pharmacy. 2022; 10(5):106. DOI: https://doi.org/10.3390/pharmacy10050106.
CrossRef - Ventola CL. The drug shortage crisis in the United States: causes, impact, and management strategies. Pharmacy and Therapeutics. 2011; 36(11):740.
- Chen T, Li J, Chen T, Sun CC, Zheng Y. Tablets of multi-unit pellet system for controlled drug delivery. Journal of Controlled Release. 2017; 262:222-31. DOI: https://doi.org/10.1016/j.jconrel.2017.07.043.
CrossRef - Thio DR, Heng PW, Chan LW. MUPS Tableting—Comparison between Crospovidone and Microcrystalline Cellulose Core Pellets. 2022; 14(12):2812. DOI: https://doi.org/10.3390/ pharmaceutics14122812.
CrossRef - RoshanRai R, Chirra P, Thanda V. Fast dissolving tablets: A novel approach to drug delivery–A Review. International journal of preclinical and pharmaceutical research. 2012;3(1):23-32.
- Maheshwari S, Singh A, Varshney AP, Sharma A. Advancing oral drug delivery: The science of fast dissolving tablets (FDTs). Intelligent Pharmacy. 2024; 2(4):580-587. DOI: https://doi.org/10.1016/ j.ipha.2024.01.011.
CrossRef - Baryakova TH, Pogostin BH, Langer R, McHugh KJ. Overcoming barriers to patient adherence: the case for developing innovative drug delivery systems. Nature Reviews Drug Discovery. 2023; 22(5):387-409. DOI: https://doi.org/10.1038/s41573-023-00670-0.
CrossRef - Kállai-Szabó N, Farkas D, Lengyel M, Basa B, Fleck C, Antal I. Microparticles and multi-unit systems for advanced drug delivery. European Journal of Pharmaceutical Sciences. 2024; 194:106704. DOI: https://doi.org/10.1016/j.ejps.2024.106704.
CrossRef - Zakowiecki D, Frankiewicz M, Hess T, Cal K, Gajda M, Dabrowska J, Kubiak B, Paszkowska J, Wiater M, Hoc D, Garbacz G. Development of a biphasic-release multiple-unit pellet system with diclofenac sodium using novel calcium phosphate-based starter pellets. 2021; 13(6):805. DOI: https://doi.org/10.3390/pharmaceutics13060805.
CrossRef - Tang ES, Chan LW, Heng PW. Coating of multiparticulates for sustained release. American Journal of Drug Delivery. 2005; 3:17-28. DOI: https://doi.org/10.2165/00137696-200503010-00003.
CrossRef - Dwibhashyam VM, Ratna JV. Key formulation variables in tableting of coated pellets. Indian journal of pharmaceutical sciences. 2008; 70(5):555. DOI: https://doi.org/10.4103/0250-474X.45391.
CrossRef - Nicklasson F, Johansson B, Alderborn G. Tabletting behaviour of pellets of a series of porosities—a comparison between pellets of two different compositions. European journal of pharmaceutical sciences. 1999; 8(1):11-7. DOI: https://doi.org/10.1016/S0928-0987(98)00056-6.
CrossRef - Kranz H, Jürgens K, Pinier M, Siepmann J. Drug release from MCC-and carrageenan-based pellets: experiment and theory. European Journal of Pharmaceutics and Biopharmaceutics. 2009; 73(2):302-9. DOI: https://doi.org/10.1016/j.ejpb.2009.05.007.
CrossRef - Thommes M, Kleinebudde P. Use of κ-carrageenan as alternative pelletisation aid to microcrystalline cellulose in extrusion/spheronisation. II. Influence of drug and filler type. European Journal of Pharmaceutics and Biopharmaceutics. 2006; 63(1):68-75. DOI: https://doi.org/10.1016/j.ejpb.2005.10.002.
CrossRef - Tunón Å, Alderborn G. Granule deformation and densification during compression of binary mixtures of granules. International journal of pharmaceutics. 2001; 222(1):65-76. DOI: https://doi.org/10.1016/S0378-5173(01)00686-X.
CrossRef - Abdul S, Chandewar AV, Jaiswal SB. A flexible technology for modified-release drugs: multiple-unit pellet system (MUPS). Journal of controlled release. 2010; 147(1):2-16. DOI: https://doi.org/10.1016/j.jconrel.2010.05.014.
CrossRef - Tumuluru JS, Fillerup E, Kane JJ, Murray D. Advanced imaging techniques to understand the impact of process variables on the particle morphology in a corn stover pellet. Chemical Engineering Research and Design. 2020; 161:130-45. DOI: https://doi.org/10.1016/j.cherd.2020.07.002.
CrossRef - Tofiq M, Persson AS, Lazorova L, Nordström J, Alderborn G. The interplay between compression mechanisms and compaction pressure in relation to the loss of tabletability of dry granulated particles. Powder Technology. 2025; 452:120519. DOI: https://doi.org/10.1016/j.powtec.2024.120519.
CrossRef - Pai R, Kohli K, Shrivastava B. Compression and evaluation of extended release matrix pellets prepared by the extrusion/spheronization process into disintegrating tablets. Brazilian Journal of Pharmaceutical Sciences. 2012; 48:117-29. DOI: https://doi.org/10.1590/S1984-82502012000100014.
CrossRef - Borandeh S, van Bochove B, Teotia A, Seppälä J. Polymeric drug delivery systems by additive manufacturing. Advanced drug delivery reviews. 2021; 173:349-73. DOI: https://doi.org/10.1016/j.addr.2021.03.022.
CrossRef - Dashevsky A, Kolter K, Bodmeier R. Compression of pellets coated with various aqueous polymer dispersions. International journal of pharmaceutics. 2004; 279(1-2):19-26. DOI: https://doi.org/10.1016/j.ijpharm.2004.03.019.
CrossRef - Wulff R, Leopold CS. Coatings from blends of Eudragit® RL and L55: A novel approach in pH-controlled drug release. International Journal of Pharmaceutics. 2014; 476(1-2):78-87. DOI: https://doi.org/10.1016/j.ijpharm.2014.09.023.
CrossRef - Lecomte F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. Blends of enteric and GIT-insoluble polymers used for film coating: physicochemical characterization and drug release patterns. Journal of controlled release. 2003; 89(3):457-71. DOI: https://doi.org/10.1016/S0168-3659(03)00155-X.
CrossRef - Thakral S, Thakral NK, Majumdar DK. Eudragit®: a technology evaluation. Expert opinion on drug delivery. 2013; 10(1):131-49. DOI: https://doi.org/10.1517/17425247.2013.736962.
CrossRef - Patra CN, Priya R, Swain S, Jena GK, Panigrahi KC, Ghose D. Pharmaceutical significance of Eudragit: A review. Future Journal of Pharmaceutical Sciences. 2017; 3(1):33-45. DOI: https://doi.org/10.1016/j.fjps.2017.02.001.
CrossRef - Drašković M, Medarević D, Aleksić I, Parojčić J. In vitro and in vivo investigation of taste-masking effectiveness of Eudragit E PO as drug particle coating agent in orally disintegrating tablets. Drug development and industrial pharmacy. 2017; 43(5):723-31. DOI: https://doi.org/10.1080/03639045.2016.1220572.
CrossRef - Punia A, Biyyala V, Faassen F, Ash J, Lamm MS. Detrimental effect of the film coat chemistry and thickness on the physical stability of amorphous solid dispersions in tablet formulations. Journal of Pharmaceutical Sciences. 2023; 112(3):708-17. DOI: https://doi.org/10.1016/j.xphs.2022.09.013.
CrossRef - Muselík J, Dvorackova K, Krejcova K, Rabiskova M, Pazourek J, Marton S, Drackova M, Vorlová L. Pellet coating thickness determination by near-infrared reflectance spectroscopy: comparison of two reference methods. Current Pharmaceutical Analysis. 2010; 6(4):225-33. DOI: https://doi.org/10.2174/157341210793292419.
CrossRef - Irfan M, Ahmed AR, Kolter K, Bodmeier R, Dashevskiy A. Curing mechanism of flexible aqueous polymeric coatings. European Journal of Pharmaceutics and Biopharmaceutics. 2017; 115:186-96. DOI: https://doi.org/10.1016/j.ejpb.2017.02.012.
CrossRef - Osei-Yeboah F, Lan Y, Sun CC. A top coating strategy with highly bonding polymers to enable direct tableting of multiple unit pellet system (MUPS). Powder Technology. 2017; 305:591-6. DOI: https://doi.org/10.1016/j.powtec.2016.10.039.
CrossRef - Vieira MG, Da Silva MA, Dos Santos LO, Beppu MM. Natural-based plasticizers and biopolymer films: A review. European polymer journal. 2011; 47(3):254-63. DOI: https://doi.org/10.1016/j.eurpolymj.2010.12.011.
CrossRef - Łunio R, Sawicki W. Influence of the components of Kollicoat SR film on mechanical properties of floating pellets from the point of view of tableting. Die Pharmazie-An International Journal of Pharmaceutical Sciences. 2008; 63(10):731-5. DOI: https://doi.org/10.1691/ph.2008.8096.
- Rujivipat S, Bodmeier R. Moisture plasticization for enteric Eudragit® L30D-55-coated pellets prior to compression into tablets. European journal of pharmaceutics and biopharmaceutics. 2012; 81(1):223-9. DOI: https://doi.org/10.1016/j.ejpb.2012.01.003.
CrossRef - Xu M, Heng PW, Liew CV. Formulation and process strategies to minimize coat damage for compaction of coated pellets in a rotary tablet press: A mechanistic view. International journal of pharmaceutics. 2016; 499(1-2):29-37. DOI: https://doi.org/10.1016/j.ijpharm.2015.12.068.
CrossRef - Moutaharrik S, Palugan L, Cerea M, Filippin I, Maroni A, Gazzaniga A, Foppoli A. Cushion-coated pellets for tableting without external excipients. International Journal of Pharmaceutics. 2024; 653:123874. DOI: https://doi.org/10.1016/j.ijpharm.2024.123874.
CrossRef - Ando M, Kojima S, Ozeki Y, Nakayama Y, Nabeshima T. Development and evaluation of a novel dry-coated tablet technology for pellets as a substitute for the conventional encapsulation technology. International journal of pharmaceutics. 2007 May 4;336(1):99-107. DOI: https://doi.org/10.1016/j.ijpharm.2006.11.042.
CrossRef - Lundqvist ÅE, Podczeck F, Newton JM. Influence of disintegrant type and proportion on the properties of tablets produced from mixtures of pellets. International journal of pharmaceutics. 1997; 147(1):95-107. DOI: https://doi.org/10.1016/S0378-5173(96)04800-4.
CrossRef - Vergote GJ, Kiekens F, Vervaet C, Remon JP. Wax beads as cushioning agents during the compression of coated diltiazem pellets. European Journal of Pharmaceutical Sciences. 2002; 17(3):145-51. DOI: https://doi.org/10.1016/S0928-0987(02)00164-1.
CrossRef - Sántha K, Kállai-Szabó N, Fülöp V, Jakab G, Gordon P, Kállai-Szabó B, Balogh E, Antal I. Comparative evaluation of pellet cushioning agents by various imaging techniques and dissolution studies. AAPS PharmSciTech. 2021; 22:1-10. DOI: https://doi.org/10.1208/s12249-020-01902-x.
CrossRef - Elsergany RN, Chan LW, Heng PW. Influence of the porosity of cushioning excipients on the compaction of coated multi-particulates. European Journal of Pharmaceutics and Biopharmaceutics. 2020; 152:218-28. DOI: https://doi.org/10.1016/j.ejpb.2020.05.015.
CrossRef - Dreu R, Ilić I, Srčič S. Development of a multiple-unit tablet containing enteric-coated pellets. Pharmaceutical Development and Technology. 2011; 16(2):118-26. DOI: https://doi.org/10.3109/10837450903499382.
CrossRef - Debunne A, Vervaet C, Mangelings D, Remon JP. Compaction of enteric-coated pellets: influence of formulation and process parameters on tablet properties and in vivo evaluation. European journal of pharmaceutical sciences. 2004; 22(4):305-14. DOI: https://doi.org/10.1016/j.ejps.2004.03.017.
CrossRef - Nguyen C, Christensen JM, Ayres JW. Compression of coated drug beads for sustained release tablet of glipizide: formulation, and dissolution. Pharmaceutical Development and Technology. 2014; 19(1):10-20. DOI: https://doi.org/10.3109/10837450.2012.751402.
CrossRef - Sawicki W, Mazgalski J. Hot tableting as a new method for obtaining tablets from slow release-coated pellets. Drug development and industrial pharmacy. 2009 ;35(7):857-65. DOI: https://doi.org/10.1080/03639040802680248.
CrossRef - Pan X, Huang Y, Dong Y, Wang Z, Zhu C, Li G, Chen B, Wu C. Process investigation of a novel compaction technique with pellet-containing granules. Therapeutic Innovation & Regulatory Science. 2013; 47(5):593-601. DOI: https://doi.org/10.1177/2168479013495681.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





