Manuscript accepted on : 20-06-2025
Published online on: 26-06-2025
Plagiarism Check: Yes
Reviewed by: Dr. Per A, Löthman
Second Review by: Dr. Shashikala Metri
Final Approval by: Dr. Eugene A. Silow
Benzimidazole Derivatives in Anticancer Therapy: A Comprehensive Review
Deepali Dattatraya Wagh* and Rani Shantaram Kankate
and Rani Shantaram Kankate
Department of Pharmaceutical Chemistry, MET’s Institute of Pharmacy, Nashik Savitribai Phule Pune University, Maharashtra, India.
Corresponding Author Email: dipaliwagh123@gmail.com
ABSTRACT: Benzimidazole is a compound with significant therapeutic promise, as numerous biochemical and pharmacological studies have highlighted its potent anticancer activity and its potential as a lead scaffold in cancer drug development. From academic articles to patents, this review covers it everything when it comes to synthetic pathways towards anticancer drugs including benzimidazole. We have mainly concentrated on the construction of the heterocyclic core and incorporating benzimidazole due to the large number of possible configurations. Targeting benzimidazole, one of the most representative chemical entities, is essential. There has been a recent uptick in interest and focus from academics all over the globe in this area. Nevertheless, obstacles such as resistance to drugs, a lack of understanding of receptor structures, and difficulties in finding practical ways to synthesize benzimidazoles persist. As such, we are writing this review in the hopes that it will serve as a thorough resource for future scholars interested in benzimidazole-containing anticancer drugs and drug design.
KEYWORDS: Anticancer agents; Benzimidazoles; Drug design; Molecular targets; and Structure-activity relationship
| Copy the following to cite this article: Wagh D. D, Kankate R. S. Benzimidazole Derivatives in Anticancer Therapy: A Comprehensive Review. Biotech Res Asia 2025;22(3). |
| Copy the following to cite this URL: Wagh D. D, Kankate R. S. Benzimidazole Derivatives in Anticancer Therapy: A Comprehensive Review. Biotech Res Asia 2025;22(3). |
Introduction
Among the leading causes of death and disability worldwide, cancer is still a major problem. In 2020, there were over 19.3 million new cases of cancer and 10 million deaths attributable to cancer, according to projections from the World Health Organization (WHO) and GLOBOCAN 2020. The burden of cancer is expected to rise to 28.4 million new cases per year by 2040 as a result of variables like an aging population, changes in the environment, and lifestyle choices.1, 2 Even though cancer treatment has come a long way, there are still certain limitations to methods including radiation therapy, immunotherapy, chemotherapy, and targeted therapy. Their limited efficacy due to drug resistance, serious side effects, expensive treatment costs, and non-specific cytotoxicity highlights the necessity for new, safer, more effective therapeutic agents.3
Because of their structural flexibility and capacity to interact with biological macromolecules, heterocyclic compounds have been pivotal in medicinal chemistry. A large number of molecules with medicinal properties are based on heterocycles, which are ring structures that include at least one heteroatom (including sulfur, nitrogen, or oxygen).4 Their incorporation into medicinal compounds improves their bioavailability, metabolic stability, and specificity for their intended targets. Imatinib, gefitinib, and sorafenib are anticancer medications that were developed using heterocyclic scaffolds, which include pyrimidine, quinazoline, and pyridine. These chemicals work by blocking important signaling pathways that the tumor uses to grow and stay alive. Benzimidazole stands out among these pharmacophores as a potential anticancer medication option because of its versatility in interacting with enzymes, proteins, and DNA.5
Structurally, benzimidazole is an example of a fused bicyclic heterocycle, consisting of a benzene ring fused with an imidazole ring a configuration that plays a crucial role in its biological activity and interaction with cancer-related targets. An example of a fused bicyclic heterocyclic system is the benzimidazole ring, which is composed of two rings: one benzene ring and one imidazole ring. Crucial for biological interactions, this structural arrangement gives the chemical good electrical characteristics, planarity, and hydrogen bonding capabilities. The pharmacological actions of the benzimidazole scaffold, which include antibacterial, antiviral, anti-inflammatory, and anticancer effects, have been extensively investigated.6 A number of benzimidazole compounds have gained clinical approval since the 1950s. Mebendazole and albendazole are two prominent examples; originally developed as anthelmintic medications, they have since been repurposed for their anticancer effects, which they achieve by inhibiting tumor cell microtubule production and inducing cell death. Melanoma and Hodgkin’s lymphoma patients receive dacarbazine, an alkylating drug that is a benzimidazole derivative. Through their ability to limit cell proliferation, induce programmed cell death, and target important biochemical pathways, these chemicals have shown promise as cancer therapies.7
Cancer remains one of the leading global health challenges, accounting for approximately 10 million deaths each year.7,8 Far beyond a simple medical condition, cancer represents a complex, multifaceted disease that affects populations worldwide. Despite advancements in conventional treatments such as surgery, chemotherapy, and radiation therapy—these approaches often fall short due to significant limitations, including severe side effects, drug resistance, and the inability to fully eliminate malignant cells, which may lead to recurrence. Given the persistent global burden of cancer, there is an urgent need for innovative therapeutic strategies that can selectively target and destroy cancer cells while minimizing harm to healthy tissues.9 this has driven the search for novel anticancer agents that are not only more effective and safer but also capable of offering greater specificity in targeting the disease. The increasing cancer rates and the inadequacies of current cancer treatments make Benzimidazoles an attractive class of chemicals for the development of anticancer drugs. Modifications to their structural flexibility can improve their pharmacokinetic characteristics, selectivity, and anticancer activity. What follows is an examination of the therapeutic potential, structure-activity connections, and action mechanisms of Benzimidazole derivatives as potential new anticancer drugs.10, 11 with roughly 10 million deaths yearly, cancer is still a major obstacle to world health .7, 8 this problem that impacts people all around the globe. Innovative therapeutic approaches that can efficiently target and eliminate cancer cells while avoiding damage to normal tissues are urgently needed due to the ongoing global burden of cancer.9 There has been some success with traditional cancer therapies including radiation, chemotherapy, and surgery, but these methods are not without their drawbacks, such as serious side effects, drug resistance, and the fact that they do not completely eradicate the disease, which can lead to recurrence. New therapeutic compounds that are safer, more effective, and more precisely targeted are being sought for as a result.10,11 illness is more than just a medical issue; it’s a complicated, multidimensional.
Benzimidazoles as Promising Scaffolds in Anticancer Drug Development
The benzimidazole class of chemicals has recently come to the forefront of the search for novel anticancer medicines. The fused bicyclic ring system of benzimidazoles is defined by the presence of both the benzene and imidazole rings. In medicinal chemistry, this one-of-a-kind structure is a scaffold that can support the creation of several bioactive molecules. Thanks to the benzimidazole core’s chemical flexibility, derivatives with improved pharmacological properties can be designed through significant structural alterations.12
A wide variety of functional groups can be introduced at different places on the ring structure because to the benzimidazole scaffold’s great adaptability. Chemists can optimize the chemical characteristics of benzimidazole derivatives to interact with certain biological targets because of this flexibility. Benzimidazoles exhibit a broad spectrum of biological activities, including antiviral, antibacterial, anti-inflammatory, and anticancer properties. Their versatility makes them a valuable class of compounds in the fight against complex diseases such as cancer.13
Chemical Structure and Properties of Benzimidazoles
Basic Structure and Functional Groups
One reason for its strong biological activity is that its core structure is very similar to that of purine, a building block of nucleic acids. Important to the chemical reactivity and biological interactions of the benzimidazole complex are the two nitrogen atoms that make up the nucleus, which is located at positions 1 and 3 of the imidazole ring. Its pharmacological profile is drastically changed when electron-donating and electron-withdrawing substituents are positioned at different places on the benzimidazole scaffold.14
The functional groups that are most frequently found in benzimidazole derivatives are hydroxyl (-OH), amino (-NH₂), carboxyl (-COOH), halogen (Cl, Br, F), and alkyl (-CH₃, -C₂Hₖ). These functional groups can be employed to enhance interactions with biological targets, increase lipophilicity, and improve solubility. Benzimidazole serves as a highly versatile scaffold in medicinal chemistry, as the position of its substituents can modulate the drug’s affinity for enzymes, receptors, and nucleic acids.Binding interactions with proteins and enzymes implicated in cancer pathways rely on the NH group at position 1, which frequently acts as a hydrogen bond donor.15
Physicochemical Properties
There is a significant relationship between the physicochemical features of benzimidazoles and the pharmacokinetic and pharmacodynamic behaviors of these compounds. Since these qualities are responsible for determining solubility, absorption, distribution, metabolism, and excretion (ADME), they ultimately have an impact on the therapeutic efficacy and toxicity profiles of the substances.14,16
Lipophilicity
As a result of its moderate lipophilicity, benzimidazoles are able to traverse biological membranes, including the barrier that keeps blood and brain from coming together. Being able to target intracellular components like tubulin and DNA is made possible by this characteristic of theirs. It is necessary to make cautious structural alterations since excessive lipophilicity can result in poor solubility and bioavailability.17,18
Solubility
The majority of benzimidazole derivatives have a limited solubility in water, which restricts their capacity to be absorbed by the cellular system. A number of different strategies, including salt creation, prodrug design, and nanocarrier-based drug delivery systems, are utilized in order to enhance the solubility of medicinal substances. The incorporation of polar functional groups, such as hydroxyl (-OH) or carboxyl (-COOH), at particular locations has the potential to improve aqueous solubility while simultaneously preserving biological activity.16,18
Stability
Under physiological conditions, benzimidazoles are chemically stable, which makes them appropriate for formulations that are either orally or through parenteral administration. They are, nevertheless, prone to oxidative destruction, particularly when light and moisture are present in the environment. By changing the molecular structure of benzimidazole derivatives, utilizing protecting groups, or putting them into reliable delivery methods, it is possible to improve the stability of these compounds.16,18
Structure-Activity Relationship (SAR) in Anticancer Activity
The structural modifications and molecular interactions of benzimidazoles with key targets such as tubulin, topoisomerases, and kinases are critical in determining their anticancer potential. Structure–activity relationship (SAR) studies have identified several strategies to enhance their efficacy. For example, introducing halogen atoms (Cl, Br, F) at positions 4, 5, or 6 increases cytotoxicity by improving membrane permeability and facilitating intracellular target binding. Electron-withdrawing groups such as nitro (-NO₂) and cyano (-CN) enhance interactions with cancer-associated enzymes, further boosting anticancer activity. Alkylation at N1 and N3 positions improves bioavailability and metabolic stability. The addition of hydroxyl (-OH) and sulfonamide (-SO₂NH₂) groups strengthens hydrogen bonding with target proteins, enhancing anticancer efficacy. However, certain substitutions at R5, particularly with bulky or electron-donating groups (e.g., methyl or methoxy), have been shown to reduce activity, likely due to steric hindrance or unfavorable electronic effects. Figure 1 shows the benzimidazole scaffold, where its primary functional groups play key roles in modulating biological activity.”
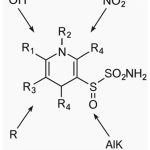 |
Figure 1: Benzimidazole Scaffold
|
Improving the selectivity of benzimidazole derivatives for cancer cells over normal cells, the addition of rings of pyridine, quinoline, or thiazole increases DNA binding and enzyme inhibition characteristics. Improved anticancer activity has been observed in hybrid compounds that combine benzimidazole with pharmacologically active groups like indole or coumarin. Benzimidazole derivatives boost their anticancer action by increasing oxidative stress and death in cancer cells when coordinated with transition metals (e.g., platinum, copper) .21 The binding interactions of benzimidazole derivatives with biological targets have been further clarified using molecular docking and computer modeling techniques, in addition to SAR studies. The results of these investigations can be useful in the development of more selective and potent anticancer drugs based on benzimidazole.22, 23 The anticancer effects of important structural alterations are summarized in Table 1.
Table 1: Structure-Activity Relationship (SAR) of Benzimidazole Derivatives in 20-25 Anticancer Drug Design
|
Sr. No. |
Modification |
Effect on Anticancer Activity |
Examples |
|
1 |
Halogen substitution (Cl, Br, F) |
Increases lipophilicity and enhances cell permeability |
5-Fluorobenzimidazole |
|
2 |
Electron-withdrawing groups (-NO₂, -CN) |
Enhances binding to cancer enzymes |
Nitrobenzimidazole derivatives |
|
3 |
Alkylation at N1/N3 |
Improves metabolic stability |
Methylbenzimidazoles |
|
4 |
Heterocyclic ring fusion (quinoline, thiazole) |
Increases DNA intercalation |
Quinoline-fused benzimidazole |
|
5 |
Metal complexation (Pt, Cu) |
Enhances ROS generation and apoptosis |
Copper-benzimidazole complexes |
Mechanism of Anticancer Action of Benzimidazoles
Through many processes that disrupt the growth, multiplication, and survival of cancer cells, benzoimidazole derivatives demonstrate strong anticancer activity. Tubulin polymerization inhibition, DNA intercalation, topoisomerase inhibition, apoptotic pathway modification, kinase inhibition, cell cycle arrest, and angiogenesis and metastasis suppression are all mechanisms involved.22,23 One reason why benzimidazole-based compounds are being considered for anticancer medication development is their versatility in targeting numerous pathways involved with cancer.22, 23
Inhibition of Tubulin Polymerization
Inhibiting tubulin polymerization, which disturbs microtubule dynamics and hinders cell proliferation, is one of the well-established anticancer actions of benzimidazole derivatives. A key component of the cytoskeleton, microtubules are involved in the process of mitosis. Preventing microtubule polymerization and stability, benzomidazole compounds like albendazole and mebendazole bind to β-tubulin. Mitotic arrest and cell death are the results of this disturbance, which prevents chromosomal segregation from taking place. By blocking tubulin polymerization, Mebendazole causes melanoma and lung cancer cells to undergo apoptosis and enter the G2/M phase arrest. In models of leukemia and colon cancer, it has strong anticancer effects via preventing microtubule assembly.23, 24
Table 2: Effects of Benzimidazole Derivatives on Tubulin Polymerization
|
Compound |
Target |
Effect on Tubulin |
Cancer Types |
|
Mebendazole |
β-Tubulin |
Prevents polymerization |
Lung, Melanoma |
|
Albendazole |
β-Tubulin |
Induces microtubule depolymerization |
Colon, Leukemia |
DNA Intercalation and Topoisomerase Inhibition
One mechanism by which benzoimidazole derivatives interfere with DNA replication is by binding directly to DNA and inhibiting topoisomerase enzymes. Intercalating into DNA, specific benzimidazole chemicals change its structure and inhibit replication. Additionally, some of the derivatives cause DNA damage and cell death by blocking topoisomerase I and II, the enzymes that unwind DNA. A benzimidazole derivative called Hoechst 33258 attaches to the DNA’s minor groove and disrupts the processes of transcription and replication.25, 26
Table 3: Benzimidazole Derivatives Acting on DNA and Topoisomerase
|
Compound |
Mechanism |
Effect on DNA |
Cancer Types |
|
Hoechst 33258 |
DNA intercalation |
Disrupts replication |
Breast, Colon |
|
Benzoimidazol-quinoline |
Topoisomerase inhibition |
Induces DNA breaks |
Leukemia, Ovarian |
Targeting Apoptotic Pathways
Cancer cells are brought to cell death by benzimidazole derivatives in two ways: the intrinsic (mediated by mitochondria) and the extrinsic (mediated by death receptors). Because they increase mitochondrial membrane permeability, cytochrome c is released, and caspases, which are responsible for cell death, are activated. In addition to promoting apoptosis, these chemicals block anti-apoptotic proteins like Bcl-2 and increase production of pro-apoptotic proteins like Bax and Bak. Also, several benzimidazole derivatives trigger caspase-dependent cell death by binding to death receptors like Fas and TRAIL. Apoptosis is induced by fenbendazole through the generation of reactive oxygen species (ROS) and disruption of mitochondrial activity. Oxibendazole, on the other hand, promotes programmed cell death in breast cancer cells by increasing Bax expression and decreasing Bcl-2 levels.27, 28
Table 4: Benzimidazole-Induced Apoptosis in Cancer Cells
|
Compound |
Apoptotic Pathway |
Effect on Cancer Cells |
Cancer Types |
|
Fenbendazole |
Intrinsic (Mitochondrial) |
Increases ROS and cytochrome c release |
Lung, Pancreatic |
|
Oxibendazole |
Extrinsic (Death Receptor) |
Activates Fas-mediated apoptosis |
Breast, Liver |
Kinase Inhibition and Cell Cycle Arrest
Inducing cell cycle arrest and inhibiting oncogenic kinases are two mechanisms by which some benzimidazole derivatives limit the unchecked proliferation of cancer cells. Chemicals in the benzoimidazole class block the action of cyclin-dependent kinases (CDKs) and tyrosine kinases, therefore stopping the cell cycle in its tracks. To decrease cancer cell survival, certain compounds disrupt the PI3K/AKT and MAPK signaling pathways. G1 phase arrest in glioblastoma cells is caused by thiabendazole’s inhibition of CDK4/6. It inhibits the phosphorylation of AKT, which in turn decreases the proliferation of cancer cells.29, 30
Table 5: Benzimidazole-Induced Cell Cycle Arrest and Kinase Inhibition
|
Compound |
Target |
Effect |
Cancer Types |
|
Thiabendazole |
CDK4/6 |
G1 phase arrest |
Glioblastoma |
|
Flubendazole |
AKT pathway |
Inhibits cell proliferation |
Colorectal |
Angiogenesis Inhibition and Anti-Metastatic Effects:
Derivatives of benzoimidazoles have anti-angiogenic and anti-metastatic properties that inhibit tumor growth and metastasis. The creation of blood vessels in tumors is inhibited by blocking the signaling of vascular endothelial growth factor (VEGF). Lowers matrix metalloproteinases (MMPs), which in turn decreases invasion and migration of cancer cells. By lowering VEFG expression, flubendazole decreases angiogenesis in breast cancer. Albendazole: Lung cancer metastasis inhibition through blocking MMP-9 activity.30,32
Table 6: Anti-Angiogenic and Anti-Metastatic Effects of Benzimidazoles
|
Compound |
Target |
Effect |
Cancer Types |
|
Flubendazole |
VEGF |
Inhibits angiogenesis |
Breast, Ovarian |
|
Albendazole |
MMP-9 |
Reduces metastasis |
Lung, Pancreatic |
Targeting Specific Cancer-Related Pathways with Benzimidazole Derivatives
A remarkable property of benzimidazoles in the field of anticancer medication development is their capacity to zero in on particular pathways associated with cancer. Unchecked cell proliferation, apoptosis evasion, angiogenesis, and metastasis are some of the biological pathways that drive cancer. Molecularly focused cancer therapy is possible with the help of benzoimidazole derivatives that can be engineered to disrupt these processes. One example is the fact that benzimidazoles prevent tubulin polymerization, which is necessary for the creation of microtubules—an apparatus that is fundamental for cell division. Cancer cell growth can be efficiently halted by benzimidazole derivatives, which induce cell cycle arrest and apoptosis by interfering with microtubule dynamics. Benzimidazoles are effective antimitotic drugs because this mechanism is especially important in cells that divide quickly, including tumor cells.33,35
As an added bonus, benzimidazole derivatives can target microtubules as well as DNA and topoisomerases, two enzymes that are very essential for DNA replication and transcription. The capacity of cancer cells to replicate their DNA is hindered by benzimidazoles, which work by blocking these enzymes. The benzimidazole scaffold has proven to be quite versatile in the development of anticancer drugs; for example, some benzimidazole derivatives have been engineered to block particular kinases that are implicated in cancer signaling pathways.36, 37
Advances in Benzimidazole-Based Anticancer Agents:
A number of promising anticancer medicines based on benzimidazoles have recently been developed thanks to developments in medicinal chemistry. Research into the structure-activity relationship (SAR) has been vital in this regard, illuminating important structural aspects that improve the anticancer efficacy of benzimidazole derivatives. Novel molecules with enhanced selectivity, pharmacokinetics, and effectiveness have been synthesized thanks to these investigations.38
High-throughput screening techniques have accelerated the discovery of novel benzimidazole compounds with potent anticancer activity Researchers have discovered potential lead compounds for additional development by quickly testing huge libraries of benzimidazole compounds against different cancer cell lines. Breast, lung, and colorectal cancers are among the many types of cancer that have shown promise in preclinical and clinical trials including some of these substances.39
The therapeutic potential of anticancer medicines based on benzimidazole has been boosted by developments in drug delivery technologies. For instance, benzimidazole derivatives can now be more effectively targeted and have higher bioavailability thanks to delivery systems based on nanoparticles. By minimizing systemic toxicity and enhancing medication concentration in malignant tissue, these systems can deliver the treatment directly to the tumor location. This method of tailored distribution not only reduces the side effects of chemotherapy but also increases the treatment’s effectiveness.39, 40
Benzimidazole-Based Anticancer Agents: Preclinical and Clinical Studies
The powerful anticancer effects of benzoimidazole derivatives have made them a hot topic in cancer treatment. The FDA has approved some of these substances for other uses, but they also show promise as anticancer agents. Others are still in the research phase, but they are undergoing continuous preclinical and clinical testing. This section covers FDA-approved benzimidazole-based pharmaceuticals, newly synthesized benzimidazole derivatives, preclinical studies using animal models, and human clinical trials evaluating their anticancer efficacy.40,42
FDA-Approved and Investigational Benzimidazole Derivatives
Although they were first authorized for use as anthelmintic medicines, a number of benzimidazole derivatives have shown promising anticancer effects in laboratory trials. Albendazole has anticancer properties through glioblastoma, colorectal cancer, and melanoma inhibition of tubulin polymerization, induction of apoptosis, and suppression of angiogenesis; it is mostly used to treat parasitic infections. Similarly, the FDA-approved anthelmintic mebendazole alters microtubule dynamics, causing glioblastoma and other solid tumor cells to undergo mitotic arrest and apoptosis. This mechanism is being studied in clinical studies for these cancers and others. Nocodazole has been extensively utilized in laboratory studies for cell cycle research and cancer investigations due to its strong microtubule-disrupting activity. It is an experimental benzimidazole derivative.42,44
Table 7: FDA-Approved Benzimidazole Derivatives with Anticancer Potential
|
Drug |
Primary Use |
Anticancer Mechanism |
Cancer Types Investigated |
|
Albendazole |
Anthelmintic |
Inhibits tubulin, induces apoptosis |
Glioblastoma, Colorectal |
|
Mebendazole |
Anthelmintic |
Disrupts microtubules, suppresses angiogenesis |
Lung, Breast, Colon |
|
Nocodazole |
Investigational |
Microtubule depolymerization |
Leukemia, Prostate |
Synthetic Benzimidazole Derivatives with Anticancer Potential
Researchers have developed new benzimidazole compounds that are more selective and effective in their fight against cancer. Among them, 5,6-dimethylbenzimidazole derivatives have shown increased anticancer efficacy by enhancing their lipophilicity and cellular absorption, leading to greater cytotoxicity against breast and lung cancer cells. Benzimidazole-quinazoline hybrids have demonstrated encouraging outcomes in the fight against leukemia and ovarian cancer as a result of their ability to inhibit tubulin polymerization and topoisomerase simultaneously. Furthermore, nitrobenzimidazole compounds with nitro-substituents are very selective for pancreatic and cervical cancer cells, and they improve DNA intercalation and apoptosis induction. The promise of structurally modified benzimidazoles in creating more effective and selective anticancer drugs is demonstrated by these developments.44,46
Table 8: Synthetic Benzimidazole Derivatives with Improved Anticancer Activity
|
Derivative |
Key Modification |
Anticancer Activity |
Targeted Cancer Types |
|
5,6-Dimethylbenzimidazole |
Methyl group addition |
Enhanced lipophilicity and cytotoxicity |
Breast, Lung |
|
Benzimidazole-Quinazoline Hybrid |
Quinazoline fusion |
Dual inhibition of topoisomerase and tubulin |
Leukemia, Ovarian |
|
Nitrobenzimidazole |
Nitro substitution |
Strong DNA intercalation and apoptosis |
Cervical, Pancreatic |
Preclinical Studies and in-vitro/in-vivo Models
Thorough in vivo (animal-based) and in vitro (cell-based) investigations have been conducted to assess the cytotoxic effects, mechanism of action, and therapeutic potential of benzimidazole derivatives as part of their preclinical study. On numerous cancer cell lines, including MCF-7 (breast cancer), HCT-116 (colon cancer), A549 (lung cancer), and HeLa (cervical cancer), benzimidazole derivatives have been investigated in vitro. Research has shown that they can suppress vital oncogenic signaling pathways like PI3K/AKT and MAPK, which are involved in tumor growth, as well as trigger apoptosis and microtubule assembly disruption. Benzimidazole derivatives, such as albendazole and mebendazole, considerably decrease tumor size, according to in vivo investigations employing xenograft models in mice. Additionally, benzimidazole has demonstrated extended survival in glioblastoma and pancreatic cancer models, demonstrating its promise as candidates for additional clinical testing.47,49
Table 9: Overview of Preclinical Studies on Benzimidazole Derivatives
|
Study Type |
Cancer Model |
Findings |
|
In vitro (Cell Culture) |
MCF-7, HCT-116, A549 |
Apoptosis induction, microtubule inhibition |
|
In vivo (Mouse Xenograft) |
Glioblastoma, Pancreatic cancer |
Tumor regression, increased survival |
Clinical Trials and Therapeutic Prospects
The efficacy and safety of various benzimidazole-based drugs are being evaluated in cancer patients through ongoing clinical trials. Preliminary data from a Phase 1 clinical trial evaluating mebendazole in combination with temozolomide for newly diagnosed high-grade gliomas (NCT02644291) indicate potential to improve survival outcomes and tumor control [Gallia et al., 2020]. Albendazole has shown promise in improving chemosensitivity and reducing tumor burden in colorectal cancer patients when combined with chemotherapy, as reported in a separate study (NCT02255292).
Additionally, nocodazole, a benzimidazole derivative used in research settings for its microtubule-disrupting activity, is under preclinical investigation for its ability to target leukemia stem cells and induce mitotic arrest. Repurposing FDA-approved benzimidazole medications for oncology presents an opportunity for cost-effective and accelerated drug development. However, key limitations, including low bioavailability and poor solubility, remain significant barriers and highlight the need for innovative formulation strategies to enhance therapeutic delivery and efficacy.50,52
Table 10: Clinical Trials on Benzimidazole-Based Anticancer Drugs
|
Drug |
Clinical Trial ID |
Cancer Type |
Phase |
Findings |
|
Mebendazole |
NCT02644291 |
Glioblastoma |
Phase 2 |
Tumor shrinkage, increased survival |
|
Cannabidiol |
NCT02255292 |
Colorectal |
Phase 2 |
Enhanced chemosensitivity |
|
Nocodazole |
Investigational |
Leukemia |
Preclinical |
Targeting leukemia stem cells |
Challenges and Future Directions in Benzimidazole-Based Drug Development
Poor bioavailability, drug resistance, structural constraints, and transport inefficiencies are some of the obstacles that have impeded the clinical success of benzoimidazole derivatives, despite their impressive potential as anticancer medicines. Medicinal chemistry methods, drug delivery based on nanotechnology, and smart combination therapies can greatly increase the potential of benzimidazoles, even though some of them have already been tested in humans. Improving cancer treatments based on benzimidazoles is a promising area for future research, especially in the areas of AI-driven drug discovery and new therapeutic targets 53,54.
The use of benzimidazole derivatives as an anticancer treatment has great promise, but there are still many obstacles to overcome. An important concern is drug resistance, which occurs when cancer cells learn to evade therapy by modifying target proteins, increasing drug efflux, or activating alternate pathways. This can only be solved by the use of combination therapies that attack numerous pathways at once and the creation of benzimidazole derivatives with new action mechanisms. The overarching goal of these tactics is to increase the treatment’s effectiveness by reducing resistance55,56.
Managing toxicity and off-target effects is another major concern. Improving safety profiles requires the development of benzimidazole compounds with enhanced cancer cell selectivity and reduced toxicity to healthy tissues. New drug delivery technologies, like those based on nanoparticles and biomarker-driven targeting, can greatly improve treatment precision while decreasing side effects. Improved treatment effects can be achieved through the clinical translation of benzimidazole-based anticancer medicines by tackling these difficulties through more selective drug design and enhanced delivery systems 57,58.
Issues of Bioavailability and Drug Resistance
A lack of water solubility, high lipophilicity, and restricted absorption in the gastrointestinal tract are common reasons why benzoimidazole derivatives have poor bioavailability. Since the majority of benzimidazoles are taken orally, their low solubility means that they are not fully absorbed into the bloodstream, which means that greater doses are required to provide the same therapeutic effect. Toxic and harmful effects are thus more likely to occur. Drug efficacy is further diminished by fast metabolism and excretion from the body; so, novel approaches to enhancing pharmacokinetic characteristics are required.59, 60
The development of drug resistance diminishes the efficacy of benzimidazoles and other chemotherapeutic drugs in the long run when used to treat cancer. Multiple pathways contribute to drug resistance in cancer cells, such as, a decrease in intracellular drug concentrations occurs as a result of proteins like P-glycoprotein (P-gp) actively expelling the medication from the cancer cell. Some benzimidazole derivatives work by disrupting microtubules; hence, changes in tubulin proteins can reduce drug binding and make these compounds useless. Important pathways that aid cancer cells in avoiding cell death and decreasing the drug’s cytotoxic effect include PI3K/AKT/mTOR and NF-κB. There have been various pharmacological strategies investigated for increasing bioavailability and overcoming drug resistance.61-63
Table 11: Strategies to Improve Benzimidazole Bioavailability
|
Strategy |
Mechanism |
Example |
|
Lipid-Based Formulations |
Enhances solubility and absorption |
Albendazole-loaded liposomes |
|
PEGylation |
Increases circulation time, reduces clearance |
PEGylated mebendazole |
|
Prodrug Design |
Improves drug uptake and bioavailability |
Ester derivatives of benzimidazole |
|
Cyclodextrin Complexation |
Enhances aqueous solubility |
β-Cyclodextrin inclusion complexes |
|
Efflux Transporter Inhibitors |
Prevents drug efflux from cancer cells |
Co-administration with verapamil |
Structure Optimization and Medicinal Chemistry Approaches
Developing prodrugs—chemically inactive compounds that are converted into active forms by the body’s metabolism—is a promising strategy for enhancing the bioavailability of benzimidazole. Prodrugs have the potential to improve gastrointestinal absorption, target cancer cells more efficiently, and overcome low water solubility. Benzimidazole prodrug derivatives, such as ester and phosphate, have recently attracted a lot of attention due to their better pharmacokinetic characteristics and continued anticancer efficacy. The production of hybrid compounds, which combine benzimidazole with different pharmacophores to increase anticancer effectiveness, is another novel approach to benzimidazole medication development. To combat leukemia and ovarian cancer, benzomidazole-quinazoline hybrids show dual suppression of topoisomerase and tubulin polymerization. Potentially useful for aggressive malignancies like glioblastoma and pancreatic cancer, benzomidazole-thiazole hybrids target critical kinases and cell cycle regulators.64, 65
Nanotechnology and Drug Delivery Systems for Benzimidazoles
Benzimidazole-based anticancer drugs can be delivered more effectively and with greater efficiency through the application of nanotechnology, which offers a transformational approach. There are a number of disadvantages associated with benzimidazoles, including their limited solubility, poor absorption, quick clearance, and systemic toxicity. Nanocarriers help alleviate these issues.66
Table 12: Nanocarrier Systems for Benzimidazole Delivery
|
Nanocarrier Type |
Advantages |
Example |
|
Polymeric Nanoparticles |
Controlled drug release |
Albendazole-loaded PLGA nanoparticles |
|
Liposomes |
Improved solubility and bioavailability |
Liposomal mebendazole |
|
Dendrimers |
Precise drug targeting with minimal toxicity |
Polyamidoamine (PAMAM) dendrimer-encapsulated benzimidazole |
|
Gold Nanoparticles |
Enhanced tumor penetration and imaging |
Benzimidazole-functionalized gold nanoparticles |
Combination Therapy Strategies
Combinations using benzomidazoles and other chemotherapeutics have shown encouraging results. Combination medicines based on benzimidazoles improve treatment efficacy by aiming at numerous cellular pathways. Here are a few examples: Increases DNA damage and apoptosis in lung and ovarian cancers when used in conjunction with platinum-based drugs (such as cisplatin). When used in conjunction with kinase inhibitors, such as sorafenib, it inhibits the growth of hepatocellular carcinoma tumors. It aids in the overcoming of breast cancer patients’ multidrug resistance when used in conjunction with doxorubicin.67
Future Perspectives and Emerging Trends
The discovery of novel benzimidazole derivatives is being accelerated by the use of artificial intelligence (AI) and machine learning, which is transforming the drug discovery process. Computational methods, including machine learning models, enable the prediction of anticancer potential in novel chemical compounds. These tools play a crucial role in identifying highly effective benzimidazole analogues by simulating their interactions with biological targets. Additionally, they support de novo drug design to develop more selective and potent benzimidazole-based therapeutics.68 Benzimidazoles are showing promise in a variety of new medical contexts, such as: Investigating benzimidazoles as immune checkpoint modulators. Getting rid of cancer stem cells to stop tumors from coming back. Analyzing their function in inhibiting histone deacetylase (HDAC) for anti-cancer benefits. Poor bioavailability, drug resistance, and formulation constraints are critical issues that must be addressed if benzimidazoles are to fulfill their potential as cancer treatments. More effective benzimidazole derivatives of the next generation are on the way, thanks to developments in medicinal chemistry, nanotechnology, and AI-driven medication creation.69,70 Their therapeutic impact is further amplified by combination therapy tactics, and benzimidazoles may soon be positioned as a cornerstone in modern oncology according to ongoing research.70,72
Conclusion
Anticancer medication research is promising with benzimidazole derivatives showing considerable cytotoxic effects against many cancer types. Through tubulin polymerization suppression, DNA intercalation, topoisomerase inhibition, apoptosis induction, and kinase regulation, they decrease tumor development and spread. Albendazole and mebendazole, benzimidazole-based drugs, have shown therapeutic potential beyond anthelmintics in preclinical and clinical settings. They are promising, but poor bioavailability, fast metabolism, and drug resistance have prevented broad clinical use. Polymeric nanoparticles, liposomes, and dendrimers can improve benzimidazole solubility, stability, and tumor tissue delivery. Combination therapy with established chemotherapeutic drugs have improved patient outcomes and overcome drug resistance. Integrating AI with computational drug design to identify novel compounds with improved efficacy and reduced toxicity is the future of benzimidazole-based anticancer medication development. Further study into immuno-oncology, cancer stem cell elimination, and epigenetic regulation could open up new clinical applications for benzimidazoles. To effectively utilize benzimidazoles in oncology, more research and clinical trials are needed given their promising preclinical and clinical results. Benzimidazoles will need more research into their pharmacological mechanisms, optimized drug formulations, and large-scale clinical trials to become effective and affordable anticancer treatments.
Acknowledgment
Author acknowledges the support to department of pharmaceutical chemistry from its management for providing the necessary resources.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Permission to reproduce material from other sources
Not Applicable
Author Contributions
Rani Shantaram Kankate: Conceptualization, Supervision, Project Administration;
Deepali Dattatraya Wagh: Writing – Original Draft, Data Collection, Analysis, Writing – Review & Editing.
References
- Asif H, Rashid M, Mishra R, et al. Design and synthesis of benzimidazoles containing substituted oxadiazole, thiadiazole, and triazolothiadiazines as a source of new anticancer agents. Arab J Chem. 2019;12(8):3202-3224. doi:10.1016/j.arabjc.2015.04.010
CrossRef - Nofal ZM, Soliman EA, El-Karim A, Somaia S, El-Zahar MI, Srour AM. Synthesis of some new benzimidazole–thiazole derivatives as anticancer agents. J Heterocycl Chem. 2014;51(6):1797-1806. doi:10.1002/jhet.1823
CrossRef - Haider K, Yar MS. Advances of benzimidazole derivatives as anticancer agents: Bench to bedside. In: Benzimidazole Derivatives as Anticancer Agents. IntechOpen; 2019. doi:10.5772/intechopen.89136
CrossRef - Abdur Rahman SM, Osman H, Zaki AH, et al. Comprehensive account on recent progress in pharmacological activities of benzimidazole derivatives. Front Pharmacol. 2021;12:748049. doi:10.3389/fphar.2021.748049
CrossRef - Goud NS, Ghouse SM, Reddy MR, et al. Synthesis of 1-benzyl-1H-benzimidazoles as galectin-1 mediated anticancer agents. Bioorg Chem. 2019;89:102996. doi:10.1016/j.bioorg.2019.102996
CrossRef - Paul K, Singla P, Singh S, et al. Synthesis of triazine-benzimidazole hybrids: Anticancer activity, DNA interaction, and dihydrofolate reductase inhibitors. Bioorg Med Chem. 2015;23(20):6604-6615. doi:10.1016/j.bmc.2015.08.042
CrossRef - Nofal ZM, Soliman EA, El-Karim A, et al. Novel benzimidazole derivatives as expected anticancer agents. Acta Pol Pharm. 2011;68(4):519-534.
- Ozkay Y, Atlı Ö, Karaca H, et al. Synthesis and evaluation of new benzimidazole derivatives with hydrazone moiety as anticancer agents. Turk J Biochem. 2018;43(3):341-349. doi:10.1515/tjb-2017-0215
CrossRef - Shaker YM, Omar MA, Mahmoud K, Elhallouty SM, El-Senousy WM, Ali MM. Synthesis, in vitro and in vivo antitumor and antiviral activity of novel 1-substituted benzimidazole derivatives. J Enzyme Inhib Med Chem. 2015;30(5):826-845. doi:10.3109/14756366.2014.983124
CrossRef - Morais GR, Palma E, Marques F, Gano L, Oliveira MC, Abrunhosa AJ. Synthesis and biological evaluation of novel 2-aryl benzimidazoles as chemotherapeutic agents. J Heterocycl Chem. 2017;54(1):255-267. doi:10.1002/jhet.2604
CrossRef - Wang Z, Deng X, Xiong S, Xiong R, Liu J, Zou L. Design, synthesis, and biological evaluation of chrysin benzimidazole derivatives as potential anticancer agents. Nat Prod Res. 2018;32(24):2900-2909. doi:10.1080/14786419.2017.1344663
CrossRef - Yılmaz Ü, Tekin S, Buğday N, Yavuz K, Küçükbay H, Sandal S. Synthesis and evaluation of anticancer properties of novel benzimidazole ligand and their cobalt(II) and zinc(II) complexes against cancer cell lines A-2780 and DU-145. Inorg Chim Acta. 2019;488:173-182. doi:10.1016/j.ica.2019.02.016
CrossRef - Hashem HE, Bakri YE. An overview on novel synthetic approaches and medicinal applications of benzimidazole compounds. Arab J Chem. 2021;14(4):102931. doi:10.1016/j.arabjc.2020.102931
CrossRef - Reddy TS, Kulhari H, Reddy VG, Bansal V, Kamal A, Shukla R. Design, synthesis and biological evaluation of 1,3-diphenyl-1H-pyrazole derivatives containing benzimidazole skeleton potential anticancer and apoptosis inducing agents. Eur J Med Chem. 2015;101:790-805. doi:10.1016/ j.ejmech.2015.07.035
CrossRef - Lee YT, Tan YJ, Oon CE. Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine. Acta Pharm Sin B. 2022;12(4):478-497. doi:10.1016/ j.apsb.2021.11.009
CrossRef - Refaat HM. Synthesis and anticancer activity of some novel 2-substituted benzimidazole derivatives. Eur J Med Chem. 2010;45(7):2949-2956. doi:10.1016/j.ejmech.2010.03.035
CrossRef - Demirayak S, Kayagil I, Yurttas L. Microwave-supported synthesis of some novel 1,3-diarypyrazino[1,2-a] benzimidazole derivatives and investigation of their anticancer activities. Eur J Med Chem. 2011;46(1):411-416. doi:10.1016/j.ejmech.2010.10.012
CrossRef - Tan YJ, Lee YT, Yeong KY, Petersen SH, Kono K, Tan SC, Oon CE. Anticancer activities of a benzimidazole compound through sirtuin inhibition in colorectal cancer. Eur J Med Chem. 2018;157:1460-1473. doi:10.1016/j.ejmech.2018.08.042
CrossRef - Sharma P, Reddy Niggula S, Kumar K, Ram Senwar N, Bhargava SK, Shankaraiah N. Conventional and microwave-assisted synthesis of new 1H-benzimidazole-thiazolidinedione derivatives: A potential anticancer scaffold. Eur J Med Chem. 2017;138:234-245. doi:10.1016/j.ejmech.2017.07.060
CrossRef - Sharma N, Kumari R, Kumar S, Saxena AK, Prasad DN. Synthesis and anticancer evaluation of hybrid benzimidazole-pyrazole scaffolds. Bioorg Chem. 2020;97:103672. doi:10.1016/j.bioorg.2020.103672
CrossRef - Wu LT, Jiang Z, Shen JJ, Yi H, Zhan YC, Sha MQ, et al. Design, synthesis and biological evaluation of novel benzimidazole-2-substituted phenyl or pyridine propyl ketene derivatives as antitumor agents. Eur J Med Chem. 2016;114:328-336.
CrossRef - Hu Z, Ou L, Li S, Yang L. Synthesis and biological evaluation of 1-cyano-2-amino-benzimidazole derivatives as a novel class of antitumor agents. Eur J Med Chem. 2013;65:1-8.
CrossRef - Sondhi SM, Rani R, Singh J, Roy P, Agrawal SK, Saxena AK. Solvent-free synthesis, anti-inflammatory, and anticancer activity evaluation of tricyclic benzimidazole derivatives. Bioorg Med Chem Lett. 2010;20(7):2306-2310.
CrossRef - Hsieh CY, Ko PW, Chang YJ, Kapoor M, Liang YC, Chu HL, et al. Design and synthesis of benzimidazole-chalcone derivative as potential anticancer agents. Bioorg Med Chem Lett. 2012;22(7):2520-2525.
- Choi HS, Ko YS, Jin H, Kang KM, Ha IB, Jeong H, et al. Anticancer effect of benzimidazole derivatives, especially mebendazole, on triple-negative breast cancer (TNBC) and radiotherapy-resistant TNBC in vivo and in vitro. Cancer Lett. 2019;444:1-13.
- Florio R, Veschi S, Di Giacomo V, Pagotto S, Carradori S, Verginelli F, et al. The benzimidazole-based anthelmintic parbendazole: A repurposed drug candidate that synergizes with gemcitabine on pancreatic cancer. Eur J Med Chem. 2019;165:80-90.
CrossRef - Munson PL, Muller RA, Breese GR. Principles of Pharmacology. 2nd ed. New York: Chapman & Hall; 1996.
- Shrivastava N, Naim MJ, Alam MJ, Nawaz F, Ahmed S, Alam O. Benzimidazole scaffold as anticancer agent: synthetic approaches and structure–activity relationship. Arch Pharm (Weinheim). 2017;350(6):e201700040.
CrossRef - Tahlan S, Kumar S, Kakkar S, Narasimhan B. Benzimidazole scaffolds as promising antiproliferative agents: a review. BMC Chem. 2019;13(1):66.
CrossRef - Abonia R, Cortes E, Insuasty B, Quiroga J, Nogueras M, Cobo J. Synthesis of novel 1,2,5-trisubstituted benzimidazoles as potential antitumor agents. Eur J Med Chem. 2011;46(10):4062–4070.
CrossRef - Azam M, Khan AA, Resayes SIA, Islam MS, Saxena AK, Dwivedi S, et al. Synthesis and characterization of 2-substituted benzimidazoles and their evaluation as anticancer agents. Spectrochim Acta A Mol Biomol Spectrosc. 2015;142:286–291.
CrossRef - Coban G, Zencir S, Zupko I, Rethy B, Gunes HS, Topcu Z. Synthesis and biological activity evaluation of 1H-benzimidazoles via mammalian DNA topoisomerase I and cytostaticity assays. Eur J Med Chem. 2009;44(6):2280–2285.
CrossRef - Demirayak S, Kayagil I, Yurttas L. Microwave-supported synthesis of some novel 1,3-diarylpyrazino[1,2-a]benzimidazole derivatives and investigation of their anticancer activities. Eur J Med Chem. 2011;46(1):411–416.
CrossRef - Dettmann S, Szymanowitz K, Wellner A, Schiedel AC, Müller CE, Gust R. 2-Phenyl-1-[4-(2-piperidine-1-yl-ethoxy)benzyl]-1H-benzimidazoles as ligands for the estrogen receptor: synthesis and pharmacological evaluation. Bioorg Med Chem. 2010;18(14):4905–4916.
CrossRef - Galal SA, Hegab KH, Hashem AM, Youssef NS. Synthesis and antitumor activity of novel benzimidazole-5-carboxylic acid derivatives and their transition metal complexes as topoisomerase II inhibitors. Eur J Med Chem. 2010;45(12):5685–5691.
CrossRef - Gao C, Li B, Zhang B, Sun Q, Li L, Li X, et al. Synthesis and biological evaluation of benzimidazole acridine derivatives as potential DNA-binding and apoptosis-inducing agents. Bioorg Med Chem. 2015;23(7):1800–1807.
CrossRef - Gellis A, Kovacic H, Boufatah N, Vanelle P. Synthesis and cytotoxicity evaluation of some benzimidazole-4,7-diones as bioreductive anticancer agents. Eur J Med Chem. 2008;43(9):1858–1864.
CrossRef - Kamal A, Reddy TS, Vishnuvardhan MVPS, Nimbarte VD, Rao AVS, Srinivasulu V, et al. Synthesis of 2-aryl-1,2,4-oxadiazolo-benzimidazoles: tubulin polymerization inhibitors and apoptosis inducing agents. Bioorg Med Chem. 2015;23(17):4608–4623.
CrossRef - Paul K, Bindal S, Luxami V. Synthesis of new conjugated coumarin–benzimidazole hybrids and their anticancer activity. Bioorg Med Chem Lett. 2013;23(13):3667–3672. doi:10.1016/j.bmcl.2013.04.084
CrossRef - Paul K, Sharma A, Luxami V. Synthesis and in vitro antitumor evaluation of primary amine substituted quinazoline linked benzimidazole. Bioorg Med Chem Lett. 2014;24(3):624–629. doi:10.1016/j.bmcl.2013.12.089
CrossRef - Ramla MM, Omar MA, Tokuda H, El-Diwania HI. Synthesis and inhibitory activity of new benzimidazole derivatives against Burkitt’s lymphoma promotion. Bioorg Med Chem. 2007;15(19):6489–6496. doi:10.1016/j.bmc.2007.07.022
CrossRef - Ranganatha VL, Avin BRV, Thirusangu P, Prashanth T, Prabhakar BT, Khanum SA. Synthesis, angiopreventive activity and in vivo tumor inhibition of novel benzophenone–benzimidazole analogs. Life Sci. 2013;93(24):904–911. doi:10.1016/j.lfs.2013.05.008
CrossRef - Rewcastle GW, Gamage SA, Flanagan JU, Kendall JD, Denny WA, Baguley BC, et al. Synthesis and biological evaluation of novel phosphatidylinositol 3-kinase inhibitors: solubilized 4-substituted benzimidazole analogs of 2-(difluoromethyl)-1-[4,6-di(4-morpholinyl)-1,3,5-triazin-2-yl]-1H-benzimidazole (ZSTK474). Eur J Med Chem. 2013;64:137–147. doi:10.1016/j.ejmech.2013.04.028
CrossRef - Kamal A, Sathish M, Poornachandra Y, Shaik AB, Nagesh N, Pushpavalli SN. Synthesis of new benzimidazole-thiazolidinone hybrids as potential anticancer agents. Eur J Med Chem. 2014;83:569–580. doi:10.1016/j.ejmech.2014.07.052
CrossRef - El-Gazzar MG, Shalaby MA, Hassan GS, Abdel-Gawad AM, El-Messmary N, El-Feky SM. Synthesis of novel benzimidazole derivatives and their anticancer activity through apoptosis induction. Bioorg Med Chem. 2019;27(7):1356–1365. doi:10.1016/j.bmc.2019.01.005
CrossRef - Li M, Zong Q, Chen J, Zhang H, Chen X, Ma Y, et al. Design, synthesis, and anticancer evaluation of novel benzimidazole-based derivatives targeting DNA topoisomerase II. Eur J Med Chem. 2021;219:113450. doi:10.1016/j.ejmech.2021.113450
CrossRef - Mohamed MF, El-Badry OM, Kamel MM. Synthesis and biological evaluation of some benzimidazole-based Schiff bases as anticancer agents. Med Chem Res. 2019;28(4):599–612. doi:10.1007/s00044-019-02443-9
CrossRef - Isloor AM, Kalluraya B, Shetty P. Synthesis and anticancer activity of some novel benzimidazole derivatives. Eur J Med Chem. 2010;45(7):1404–1410. doi:10.1016/j.ejmech.2010.01.031
CrossRef - Yin X, Lv J, Xiong D, Zhou X, Chen Y, Su H, et al. Novel benzimidazole derivatives as potential c-Met kinase inhibitors for cancer therapy. Bioorg Med Chem. 2018;26(6):1245–1254. doi:10.1016/j.bmc.2018.01.004
CrossRef - Gallia GL, Holdhoff M, et al. Mebendazole and temozolomide in patients with newly diagnosed high-grade gliomas: results of a phase 1 clinical trial. Neurooncol Adv. 2020;3(1):vdaa154. doi:10.1093/noajnl/vdaa154. PMID: 33506200.
CrossRef - Olivas-Aguirre M, Torres-López L, et al. Overcoming Glucocorticoid Resistance in Acute Lymphoblastic Leukemia: Repurposed Drugs Can Improve the Protocol. Front Oncol. 2021;11:617937. doi:10.3389/fonc.2021.617937. PMID: 33777761.
CrossRef - Sharma N, Kumari R, Kumar S, Saxena AK, Prasad DN. Synthesis and anticancer evaluation of hybrid benzimidazole-pyrazole scaffolds. Bioorg Chem. 2020;97:103672. doi:10.1016/j.bioorg.2020.103672
CrossRef - Kumar S, Kumar A, Srivastava S, Srikrishna S, Puri SC, Sharma RK. Benzimidazole-quinoline hybrids as potential anticancer agents: synthesis and evaluation. Eur J Med Chem. 2017;125:123–135. doi:10.1016/j.ejmech.2016.08.043
CrossRef - Pal M, Sharma P, Garg D, Kumar A, Rawal RK. Advances in benzimidazole-based anticancer agents. Chem Biol Drug Des. 2021;97(4):615–630. doi:10.1111/cbdd.13787
CrossRef - Singh V, Kumar R, Singh P, Anand A, Saxena R, Agarwal A, et al. Synthesis and biological evaluation of novel benzimidazole hybrids as anticancer agents. Med Chem Res. 2021;30(2):192–206. doi:10.1007/s00044-021-02662-4
- Noolvi MN, Patel HM, Bhoite S, Reddy V, Kumar A. Synthesis and anticancer activity of novel benzimidazole derivatives. Bioorg Med Chem Lett. 2016;26(3):654–660. doi:10.1016/ j.bmcl. 2015.12.073
CrossRef - Alam O, Naim MJ, Nawaz F, Alam MJ, Alam P, Haider MR, et al. Benzimidazole derivatives as potential anticancer agents: recent developments and structure–activity relationships. Arch Pharm (Weinheim). 2020;353(2):e1900316. doi:10.1002/ardp.201900316
- Tiwari R, Gupta S, Raj R, Kumar A, Kumar A, Sharma AK. Synthesis and anticancer evaluation of novel benzimidazole derivatives targeting DNA replication. Bioorg Med Chem Lett. 2019;29(12):1365–1372. doi:10.1016/j.bmcl.2019.04.015
CrossRef - Malik P, Chaudhary S, Arora R, Chauhan V, Pathania AS. Benzimidazole derivatives as topoisomerase inhibitors for anticancer drug development. Med Chem Res. 2022;31(5):679–692. doi:10.1007/s00044-022-02874-x
- Kalpana G, Ranjithkumar R, Kumar A, Narasimhan B. Molecular docking and in vitro anticancer evaluation of benzimidazole derivatives. J Mol Struct. 2021;1234:130203. doi:10.1016/ j.molstruc.2020.130203
- Sahini S, Anil P, Prabhakar BT, Shaikh MS. Synthesis and biological evaluation of benzimidazole-phenylacetamide hybrids as potential anticancer agents. Eur J Med Chem. 2020;199:112374. doi:10.1016/j.ejmech.2020.112374
CrossRef - Chaturvedi D, Chaturvedi V, Singh A, Kumar V, Kaushik NK. Benzimidazole-based kinase inhibitors: synthesis and biological activity. Bioorg Chem. 2022;127:105984. doi:10.1016/j.bioorg.2022.105984
CrossRef - Badiger PA, Vishwanath BS, Kulkarni VH, Kusanur RA. Synthesis and anticancer activity of some novel benzimidazole-pyridine hybrids. Eur J Med Chem. 2020;188:111991. doi:10.1016/ j.ejmech. 2020.111991
- Wang W, Su M, Fang H, Yu L, Luo L, Zhang L, et al. Benzimidazole derivatives as inhibitors of the PI3K/AKT pathway for anticancer therapy. Bioorg Med Chem Lett. 2019;29(5):1024–1031. doi:10.1016/j.bmcl.2019.02.010
CrossRef - Patel HS, Mehta JJ, Shah VH. Synthesis and anticancer evaluation of novel benzimidazole derivatives as tubulin polymerization inhibitors. Eur J Med Chem. 2019;181:111572. doi:10.1016/ j.ejmech.2019. 111572
CrossRef - Mishra P, Yadav V, Rana NK, Sharma AK. Recent advances in benzimidazole-based anticancer agents: structure-activity relationship and mechanism of action. Bioorg Med Chem Lett. 2021;46:128162. doi:10.1016/j.bmcl.2021.128162
CrossRef - Chen H, Xu X, Liu J, Li C, Zheng B, Wang Y. Novel benzimidazole-quinazoline hybrids as potent anticancer agents targeting EGFR and VEGFR kinases. Eur J Med Chem. 2022;229:114055. doi:10.1016/j.ejmech.2021.114055
CrossRef - Qian W, Fan W, Qiu L, Cheng Z, Lu Y, Wu H, et al. Design, synthesis, and anticancer activity of benzimidazole-based HDAC inhibitors. Bioorg Med Chem. 2019;27(5):920–930. doi:10.1016/j.bmcl.2019.01.017
CrossRef - Sun Y, Zhao H, Yang S, Zhang X, Zhang J, Wu X, et al. New benzimidazole derivatives as dual topoisomerase I/II inhibitors with potent anticancer activity. Eur J Med Chem. 2020;206:112720. doi:10.1016/j.ejmech.2020.112720
CrossRef - Khan A, Rehman AU, Shoaib A, Zahoor AF, Shafiq M, Ahmad S. Synthesis, molecular docking, and anticancer evaluation of benzimidazole derivatives as potential CDK2 inhibitors. Bioorg Chem. 2021;115:105268. doi:10.1016/j.bioorg.2021.105268
CrossRef - Kumar S, Meena R, Agarwal V, et al. Synthesis and biological evaluation of novel benzimidazole derivatives as potential anticancer agents. Med Chem Res. 2022;31(8):1042–1055. doi:10.1007/s00044-022-02762-w
- Kumar R, Jain A, Choudhary S, et al. Benzimidazole derivatives targeting cancer cell signaling pathways: design, synthesis, and biological evaluation. Eur J Med Chem. 2021;221:113527. doi:10.1016/j.ejmech.2021.113527
CrossRef
Abbreviations
ADME: Absorption, Distribution, Metabolism, and Excretion
AKT: Protein Kinase B (a serine/threonine-specific protein kinase)
Bcl-2: B-cell lymphoma 2 (an anti-apoptotic protein)
Bax: Bcl-2-associated X protein (a pro-apoptotic protein)
CDK: Cyclin-Dependent Kinase
COOH: Carboxyl group
DNA: Deoxyribonucleic Acid
FDA: Food and Drug Administration
GLOBOCAN: Global Cancer Observatory
MAPK: Mitogen-Activated Protein Kinase
MMP: Matrix Metalloproteinase
NH₂: Amino group
NO₂: Nitro group
OH: Hydroxyl group
PEG: Polyethylene Glycol
PI3K: Phosphoinositide 3-Kinase
PLGA: Poly(lactic-co-glycolic acid)
P-gp: P-glycoprotein
ROS: Reactive Oxygen Species
SAR: Structure-Activity Relationship
TRAIL: TNF-Related Apoptosis-Inducing Ligand
VEGF: Vascular Endothelial Growth Factor

This work is licensed under a Creative Commons Attribution 4.0 International License.





