Manuscript accepted on : 13-06-2025
Published online on: 18-06-2025
Plagiarism Check: Yes
Reviewed by: Dr. Mahesh M
Second Review by: Dr. Parasuraman S
Final Approval by: Dr. Eugene A. Silow
Revolutionizing Burn Care: The Therapeutic Potential of Nile Tilapia Skin
Sandeep Sonawane , Harshada Suren Patil*
, Harshada Suren Patil* , Kaveri Shantaram Panpatil
, Kaveri Shantaram Panpatil , Shruti Deepak Shinde
, Shruti Deepak Shinde and Priyanka Sanjay Wabale
and Priyanka Sanjay Wabale
Department of Pharmaceutical Quality Assurance, MET’s Institute of Pharmacy, Bhujbal Knowledge City, Affiliated to Savitribai Phule Pune University, Adgaon, Nashik, India.
Corresponding author E-mail: harshadaptl2001@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3372
ABSTRACT: Tilapia fish skin is emerging as a promising and cost-effective alternative for burn treatment, offering superior healing properties compared to traditional wound dressings such as Silver Sulfadiazine and Paraffin Gauze. Rich in collagen, omega-3 fatty acids, and bioactive compounds, Tilapia skin accelerates epithelialization, with studies by Lima Júnior et al. showing healing in 9.77–18.10 days compared to 11.20–21.30 days for conventional treatments (reducing healing time by up to 25%). It also reduces pain, potentially minimizes scarring, and lowers infection rates. Unlike conventional dressings that require frequent changes, Tilapia skin remains in place for a longer period, enhancing patient comfort and reducing pain and infection risks. Comparative analysis reveals that while Silver Sulfadiazine has antibacterial properties and Paraffin Gauze is easy to use, both lead to higher pain levels, slower healing, and increased scarring due to frequent dressing changes. Tilapia skin’s accessibility and affordability make it particularly beneficial for resource-limited settings. Additional advantages include its natural analgesic effect, strong microbial resistance, and dressing-free application, reducing the need for painkillers and minimizing medical interventions. However, challenges such as the need for specialized sterilization requirements, odor sensitivity, and adherence difficulties in some anatomical regions must be addressed. As research advances, Tilapia skin holds considerable potential in burn management, offering a biocompatible, efficient, and widely accessible solution for improved wound care worldwide.
KEYWORDS: Burn; Burn Injury; Burn Treatment; Nile Tilapia Fish; Wound dressing
Download this article as:| Copy the following to cite this article: Sonawane S, Patil H. S, Panpatil K. S, Shinde S. D, Wabale P. S. Revolutionizing Burn Care: The Therapeutic Potential of Nile Tilapia Skin. Biotech Res Asia 2025;22(2). |
| Copy the following to cite this URL: Sonawane S, Patil H. S, Panpatil K. S, Shinde S. D, Wabale P. S. Revolutionizing Burn Care: The Therapeutic Potential of Nile Tilapia Skin. Biotech Res Asia 2025;22(2). Available from: https://bit.ly/4kMzdo7 |
Introduction
Burn injury is a major public health issue, due to the worldwide annual occurrence of 11 million cases that cause more than 300,000 fatalities.1,2 Research indicates burn injuries should be classified as chronic diseases because their effects on the immune system probably cause long-term morbidity.3 Up to three months of hospitalization, along with permanent deformities and disability, result from the survival of nonfatal burn injuries, according to research.4 Wound management that starts soon after injury with debridement and autografting acts as standard medical practice since it minimizes the risk for sepsis along with organ dysfunction. The shortage of available autologous skin when treating extensive burns forces surgeons to select either allogeneic or xenogeneic grafts as interim dressings after performing surgical debridement. The most common origin for these short-term grafts comes from human deceased donors and porcine skin materials.5 The application of both dead human grafts and pig material poses risks for the body to reject the grafts and the transmission of infectious diseases. The prohibition against porcine grafts exists in Muslim communities due to cultural as well as religious beliefs. 5,6
History of Burns
Historical records show burn injuries have tortured human beings across multiple generations of recorded history. Archaeological records show that ancient Egypt documented the use of mud and excrement together with oil and plant extracts, as well as other substances, for treating burns, as shown in the Ebers Papyrus written around 1500 BC. In ancient Greece, Hippocrates championed the use of dressings containing pig fat along with resin and bitumen.7 During medieval times, medical interventions relied primarily on experience-based methods. Upon its arrival, the Renaissance brought back abandoned scientific investigation methods. In 1517–1588, Italian surgeon Leonardo Fioravanti achieved nose reattachment through the use of “balsama artificiato,” a pharmaceutical solution. The anecdote demonstrates medieval restoration attempts of burned tissues, which preceded contemporary grafting methods. 8 The Rialto fire of 1921 and the Coconut Grove nightclub fire of 1942 resulted in crucial progress for burn management, which became instrumental in defining contemporary perspectives on fire burn pathophysiology.9 Burn care has gained remarkable speed during the past five decades through advancements like antimicrobial wound coverings and fluid treatment protocols, and early surgical procedures with artificial skin substitute development.10 Despite these advancements, burn injuries continue to be a serious global concern, particularly in low-resource settings, where access to modern treatments is often limited.
Types of Burn Injuries and their Impact on Wound Healing
Types of Burns Based on Depth
Burns are classified based on how deeply they penetrate the skin layers, since it affects both the duration of healing and the necessary treatment approach.
Superficial (First-Degree Burns)
These burns affect only the epidermis, resulting in red, dry skin surfaces, causing pain and resembling sunburn symptoms. After cooling and hydration, the burns require healing time between 3-7 days.
Superficial Partial-Thickness (Second-Degree Burns)
These burns involve the epidermis and part of the dermis, which leads to red blistered skin with pain. The healing process takes between 10-14 days, during which time moist bandages may be needed.
Deep Partial-Thickness (Second-Degree Burns)
These burns extend deeper into the dermis, causing pale and moist tissue, which becomes painless because of nerve damage. Such burns heal between 3 and 6 weeks, yet patients very likely face both infection and scarring risks.
Full-Thickness (Third-Degree Burns)
These burns destroy the epidermis and entire dermis, resulting in white or charred, or leathery tissue with no remaining nerve function. Skin grafting of the area is a must, and there is a high risk of complications.
Full Thickness and Tissues Beneath (Fourth-Degree Burns)
The most severe type, these burns penetrate to muscles, bones, and organs, thereby creating black, dead tissue that may need potential amputation of the burned area. Patients normally require surgical measures combined with reconstructive surgery during their treatment. 11
Types of Burns Based on Cause
The nature of burn injuries depends on their origin and magnitude because this determines both therapeutic strategies and recovery results. The selection of proper interventions, together with prevention of long-term complications, depends on understanding these different burn types.
Thermal Burns
Thermal Burns develop from exposure to hot liquids that cause scalds and direct contact with flames that result in flame burns. Superficial dermal burns occur most frequently in children and elderly people when scalds affect them. The combination of deep dermal and full-thickness burns occurs with flame burns, while these injuries sometimes include damage to the respiratory system. Scalds heal more quickly than flame burns, which often need surgical treatment. 12 45
Electrical Burns
The contact with either low-voltage or high-voltage electrical sources leads to electrical burns. The wounds from electrical burns consist of entry and exit points that produce tissue damage levels which depend on the voltage strength and resistance, and the current path. The internal damage from high-voltage injuries requires ongoing observation because these wounds might develop delayed necrosis. 13
Chemical Burns
Exposure to acids, alkalis, or industrial chemicals causes chemical burns that frequently occur in workplace accidents, together with household exposures. The depth of penetration from alkali burns exceeds that of acid burns, while hydrofluoric acid stands out as a dangerous substance that causes progressive tissue destruction. Medical treatment for chemical burns includes neutralization with substances like calcium gluconate for hydrofluoric acid burns, while chemical reactions during healing delay the recovery process.14
Physiological response and complications of burn injuries
Burn injuries initiate multiple physiological processes that affect both the healing of affected tissue wounds and body-wide organ functioning. The responses triggered by burn injuries affect both how deeply tissues become damaged and the likelihood of complications and the duration of recovery. Such uncontrolled injuries result in dangerous local and systemic effects, including multiple organ failure.
Local Response to Burn Injuries
There are three distinct zones in burn injuries, each representing different degrees of tissue damage and healing potential. 15
Zone of Coagulation
This is the area of maximum damage where protein coagulation leads to complete tissue destruction.
Zone of Stasis
Surrounding the coagulation zone, this area has decreased tissue perfusion. The tissue is at risk of developing irreversible damage unless medical personnel provide correct resuscitation measures. Hypotension combined with infection or edema creates conditions that deteriorate the tissue damage in this area.
Zone of Hyperaemia
The outermost zone, which shows improved perfusion and recovery potential unless it faces severe sepsis or prolonged hypoperfusion.
Proper management of the zone of stasis prevents both widening and deepening of the wound since this area exists as three-dimensional tissue.
Systemic Response to Burn Injuries
A burn injury that affects 30% or more of the total body surface area (TBSA) triggers systemic inflammation, which results in multi-organ dysfunction.16
Cardiovascular Changes
The inflammation of capillaries leads to protein and fluid loss from blood vessels, which creates edema. The body responds by narrowing blood vessels in both the peripheral areas and the splanchnic region. Myocardial contractility decreases, possibly due to tumor necrosis factor (TNF) release. The body experiences hypotension and end-organ hypoperfusion when fluid escapes through burn wounds and when systemic blood pressure decreases.17
Respiratory Changes
The activity of inflammatory mediators creates bronchoconstriction, which raises the possibility of blocked airways. The development of adult respiratory distress syndrome (ARDS) occurs in patients with severe burn injuries. The body’s metabolic processes increase threefold during hypermetabolism, thus causing muscle deterioration alongside decreased immune function. The need for early and aggressive enteral feeding becomes essential for burn patients because it helps to reduce catabolic effects while maintaining gut integrity.18
Immunological Changes
The immune system develops a nonspecific reduction in its activity, which impacts both cell-mediated and humoral immunity. Opportunistic infections, along with sepsis, become more likely due to this condition.19
Neurological Response
Intensive burns with severe injuries lead to neurological changes that produce burn-induced encephalopathy and delirium, and altered pain processing because of extended inflammatory signals. The combination of chronic pain syndromes and post-traumatic stress disorder (PTSD) frequently affects patients who experience severe burns.20
Conventional Burn Treatments: Approaches, Limitations, and Future Considerations
Wound treatment for burns requires immediate appropriate care, which both mitigates harm and accelerates recovery. Standard treatments encompass several key interventions:
Cooling of Burned Areas
Burn victims need immediate cold application on affected areas to decrease tissue destruction and minimize discomfort. Ice or extremely cold substances should not be used in burn treatments because they can cause vasoconstriction and secondary tissue damage. Water should be used as a cooling agent at temperatures ranging from 10°C to 20°C, according to the research. 21
Fluid Resuscitation
The loss of substantial fluids through burns leads to hypovolemia before causing shock in patients. Medical practitioners use the Parkland formula, introduced in 1968, to determine fluid requirements throughout the 24-hour post-trauma period during which they administer lactated Ringer’s solution for burn shock prevention. 22
Infection Control
The risk of infection remains high for burn wounds because improper management can lead to sepsis development. Strict infection control measures, including aseptic dressing techniques and careful observation of wound development, are crucial. Doctors should use antibiotics only for confirmed infections or perioperative prophylaxis, since systemic antibiotic prevention is discouraged to stop antibiotic-resistant organisms and avoid secondary infections. 23, 24
Nutritional Support
After severe burn injury, the body enters a state of extreme catabolism, which develops through the combined effects of catecholamines, cortisol, and inflammatory cytokines. After the injury, the body starts this response within 24-48 hours until it reaches its peak at around two years. The stress response after a burn injury produces various negative effects, including muscle tissue reduction and delayed wound closure, and insulin system problems with weakened immunity function.25 Early nutritional support helps patients overcome this phase while accelerating their wound healing process and preventing infections. Wound healing and recovery strongly benefit from proper nutrition. Clinicians need to manage macronutrient intake carefully in order to prevent metabolic problems, including hyperglycemia and immunosuppression. 23,26
Surgical Intervention (Early Excision and Grafting)
The medical procedure includes immediate removal of dead tissue through early excision combined with grafting, which remains the standard therapy to advance healing while minimizing the dangers of infection. Patients receive autografts as their preferred choice because autografts use their skin, while skin grafting practices face the challenge of donor site restrictions when treating large burns. 26
Limitations of Existing Therapies
While medical progress has been made, medical treatments for burns continue to carry significant restrictions. Standard fluid resuscitation formulas fail to precisely determine individual fluid requirements, thus causing either inadequate or excessive resuscitation, which creates the risk of complications between hypoperfusion and fluid overload. Medical teams encounter enhanced difficulties in infection control because antibiotic-resistant organisms continue to spread; therefore, practitioners need to implement both antibiotics with caution alongside rigorous infection prevention standards to minimize complications. Special care must be taken to maintain proper macronutrient proportions, as excessive carbohydrates can lead to hyperglycemia, increased inflammation, decreased muscle strength, and high-fat consumption can promote lowered immunity and increased susceptibility to infections. Surgical graft procedures dealing with burn patient injuries encounter two major complications that increase the difficulty of healing following surgical reconstruction, and suffer from a scarcity of donor sites. 26
Why Tilapia Skin was Chosen for Burn Care?
Nile tilapia (Oreochromis niloticus) skin has gained recognition as an effective biomaterial dressing for burns due to its clinical applications. 27 It contains a non-infectious microbiota and a human skin-like morphological structure, with a higher composition of Type I and Type III collagen that exceeds that found in human skin.28,29 It is essential for tissue repair and faster recovery. Its tensile strength, moisture retention, and biocompatibility make it an ideal wound dressing. 29
Tilapia skin also possesses high levels of omega-3 fatty acids, which provide antioxidant and anti-inflammatory benefits, further accelerating the healing process.30 Additionally, marine peptides from tilapia skin collagen closely resemble human collagen in the extracellular matrix, containing eight essential and nine non-essential amino acids that enhance tissue compatibility.31
In Brazil, tilapia skin is a cost-effective alternative to human and pig skin, offering an affordable and widely available treatment for burns. 28 Tilapia skin is an ideal skin graft due to its superior collagen content, providing both Type I and Type III collagen at levels that exceed those found in human skin. Its tensile strength is greater than that of human skin, and it retains more moisture, making it an optimal choice for wound healing.33 Clinical studies have demonstrated that burns treated with tilapia skin heal faster, with reduced pain and fewer dressing changes, improving patient comfort.27,31 Its high moisture content and collagen composition create an optimal environment for recovery, shortening healing time by several days. Patients receive two benefits from tilapia skin dressings, i.e., decreased pain experience leading to better comfort levels and fewer dressing changes, which leads to faster healing rates. 32
With its structural similarity to human skin, superior collagen content, and therapeutic properties, tilapia skin stands out as a promising biomaterial for burn treatment.
Tilapia Skin as a Xenograft
A xenograft is a procedure of transplanting body tissue or organs between different species. In burn treatment, the application of xenografts provides brief protective coverage to burns that defends the wounds from infections and allows healing to progress. Research has proven that fish skin made from tilapia operates well as a biological dressing. This choice prevails over xenografts derived from pigs as well as cattle, and human cadavers since it addresses several concerns. Xenografts from mammalian resources increase the danger of disease transfer and provoke immune reactions within the body. The use of these dressing materials creates issues with some religious faiths and also costs higher prices than alternatives, while being monitored and not accessible in all locations.33 The properties of tilapia skin allowed it to tightly adhere to wound beds while decreasing the number of dressing changes and the amount of anaesthesia needed, which benefited patients and healthcare staff through work reduction. The use of tilted fish skin offered economical benefits as an effective burn wound treatment for superficial partial thickness injuries.34
Preparation of Tilapia Skin for Medical Use
Tilapia skin, due to its structural and biological similarity to human skin, has made it a promising alternative option for burn skin grafts. Multiple advantages occur because of tilapia skin use, primarily stopping infections and decreasing fluid loss, and improving healing times. For security and effectiveness purposes, the sterilization procedure must thoroughly remove all dangerous bacteria and contaminants from tilapia skin before its use. 35
Sterilization Methods
To make tilapia skin safe for medical applications, two primary sterilization methods are used
Chemical Sterilization
Chemical sterilization is the first step in preparing tilapia skin. Using antimicrobial agents and preservatives helps eliminate pathogens from tilapia skin without harming tissue structures. The commonly used agents include.
Chlorhexidine Di Gluconate (2%)
Chlorhexidine di gluconate exists as a 2% solution, used to sterilize tilapia skin primarily during the initial stages of the process. As a broad antimicrobial agent, chlorhexidine gluconate exhibits a broad-spectrum antimicrobial ability against both bacteria and fungi. It shows low toxicity, non-corrosive properties, as well as safe performance on biological tissues, ideal for the first stage of sterilization processes 35
Glycerol (50%, 75%, or 99%)
Acts as a preservative to preserve tissue hydration levels by preserving biological tissue structure. The prepared skin benefits from glycerol as it carries antibiotics and antifungal agents, which improve antimicrobial effectiveness. 35
Sterile Saline
Used as a gentle washing agent to remove any residual chemicals, ensuring that the skin is thoroughly cleaned and free from contaminants. It also helps in maintaining the structural integrity of the tissue. 35
Penicillin/Streptomycin/Fungisol (1%) Solution
A treatment mixture of antibiotics and antifungal agents, used for the prevention of bacterial and fungal skin contamination. This keeps the skin pathogen-free, helps reduce potential infections while supporting proper wound recovery. 35
Radio sterilization
After chemical sterilization, tilapia skin is packaged in plastic envelopes until it reaches a cobalt-60 irradiator for exposure to doses of 25 or 30 kGy. Scientific studies prove that the sterilization method preserves both the collagen structure as well as all skin properties unaltered. Microbiological testing, histological analysis, and tensiometric properties analysis show that Nile Tilapia skin procurement by chemical or radiosterilization methods prepares the skin for biological dressing usage without notable changes. 34,35
Application of Tilapia Skin in Burn Treatment
Surgical Technique
The surgical application of tilapia skin for medical purposes combines tissue from tilapia fish with wounds or skin lesions to promote new skin development. The process is described in detail below.36-38
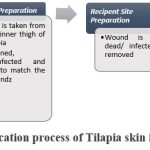 |
Figure 1: Application process of Tilapia skin in Burn Care.
|
Aftercare
The following is a description of some of the aftercare that should be followed:
Bandage
A bandage needs to be placed on top of the tilapia skin graft, both for protection and to maintain its proper position. The medical staff must check dressing placement daily to prevent either tight or loose conditions. 39
Pain control
It is common for patients to experience pain during their healing period. The physician provides medication treatments for pain relief. Individuals recovering from surgical procedures can find relief through the usage of cold compresses since they decrease pain as well as swelling. 40
Cleaning and wound care
To prevent infection, the graft site should be kept both clean and dry. Minimal infection will occur when patients keep their graft site both clean and dry. The physician will demonstrate step-by-step instructions about cleaning and dressing, replacing methods. The medical staff may use topical ointments or creams as part of the healing process.41
Avoid strenuous physical activity
The Patient needs to remain inactive with no sports or demanding activities until their physician allows them back to normal physical activity. Graft healing usually needs stability, so any unnecessary movements should be limited since they could slow down recovery time. 39
Nutrition management
The process of healing requires proper nutrition for it to happen quickly while ensuring its optimal results. A person should eat a balanced diet that includes healthy proteins, vitamins, and essential minerals. Alcohol consumption and smoking should be eliminated from a person’s regimen because they create a negative impact on healing processes. 40
Follow-up monitoring
Regular evaluations by the physician determine the patient’s healing and recovery progress. For verification of proper healing, clinical tests such as blood tests or imaging will be conducted. 41
Scientific Evidence Supporting Tilapia Skin In Burn Treatment
Research studies about Tilapia skin dressing effectiveness for burn wounds show promising outcomes that match or exceed traditional therapeutic approaches.
Comparable Healing to Standard Treatments
The Phase II randomized controlled trial (RCT) performed by Lima Júnior et al.34 during 2019 investigated Nile Tilapia skin as an occlusive xenograft for burn wound care. The research evaluated Tilapia skin against silver sulfadiazine cream as a conventional therapy for treating small burns.
The research included 62 participants while monitoring vital clinical wound parameters, including healing speed, together with pain levels and dressing requirements, and medicine consumption. Patients receiving Tilapia skin treatment healed their wounds at a faster rate compared to subjects under regular clinical protocols. Specifically, complete repithelialization occurred in:
Outpatients: 9.77 days (Tilapia skin) vs. 11.20 days (silver sulfadiazine).
Inpatients with superficial burns: 10.56 days (Tilapia skin) vs. 11.70 days (silver sulfadiazine).
Inpatients with deep partial-thickness burns: 18.10 days (Tilapia skin) vs. 21.30 days (silver sulfadiazine).
Applying Tilapia skin to burn injuries led to less necessity of dressing maintenance, which improved both patient comfort and healthcare resource availability. Patient ratings on the Visual Analogue Scale (VAS) showed lower pain intensity among subjects using Tilapia skin for burn treatment, particularly among patients with severe burns during hospital stay.34
Rapid Re-Epithelialization and Safety in Partial-Thickness Burns
In 2019, Lima Júnior et al.42 documented the implementation of Tilapia skin as a xenograft on 23-year-old gunshot survivors who experienced partial-thickness burns. The subject presented with proper upper limb superficial burns and deep partial-thickness burns on the left upper limb. Tilapia skin treatment did not need additional dressing changes due to its effective sticking properties with the wound surface. A 12-day period was necessary for superficial burn healing, alongside deep burn healing, which took 17 days. No adverse effects emerged, and the biomaterial proved its excellent compatibility with human tissue. This research demonstrates that Tilapia skin functions as an innovative burn dressing, offering both superior availability and ease of application and exercise.
Look and feel comfortable together with reduced hospital staff workload, supporting the cost-effective nature of this approach as an alternative to traditional methods of treatment. 42
Accelerated Wound Healing and Pain Reduction
The study performed by Lima Júnior et al.34 utilized a Phase II randomized controlled trial to evaluate Nile Tilapia skin xenografts for burn treatment against silver sulfadiazine cream (SSDC) as the control. A total of 62 participants took part in the research to evaluate critical clinical data points, including wound healing duration and pain ratings, as well as dressing change requirements and medicine use.
The study showed that Tilapia skin decreased healing time for every participant in the research groups:
Outpatients with superficial burns: Healing in 9.77 days (Tilapia skin) vs. 11.20 days (SSDC).
Inpatients with superficial burns: Healing in 10.56 days (Tilapia skin) vs. 11.70 days (SSDC).
Inpatients with deep partial-thickness burns: Healing in 18.10 days (Tilapia skin) vs. 21.30 days (SSDC).
The application of Tilapia skin provided additional pain relief benefits. Patients treated with Tilapia skin reported significantly lower pain scores on the Visual Analogue Scale (VAS) compared to the SSDC group, especially from the second to eighth evaluation visits for deep burn patients. Patients needed less analgesic medication, and they used considerably lower amounts of ketamine and fentanyl during the treatment period. The requirement for dressing changes decreased significantly because of the Tilapia skin application. The use of Tilapia skin required patients to experience 60-70% less dressing requirement than SSDC patients, minimizing patient discomfort and healthcare workload.
This research has established that Tilapia skin serves dual purposes by enhancing healing time while reducing pain and burn-related discomfort, so that it proves to be both a viable and cost-effective alternative to conventional burn treatments. 34
Superior Moisture Retention and Comfort
A 2022 case series authored by Putri et al. evaluated Tilapia skin xenografts against paraffin gauze for full-thickness burn dressing after burn patients underwent excisional debridement. Research conducted in Dr. Cipto Mangunkusumo Hospital’s Burn Unit of Indonesia examined four patients with 20–40% total body surface area burns who underwent excisional debridement within 96 hours post-burn. Tilapia skin xenografts needed fewer dressing modifications compared to paraffin gauze, as observed in the study results. Durational data showed that patients under Tilapia skin therapy required two fewer dressing changes during the 10-day observation period. The research confirmed both the safety and biocompatibility of Tilapia skin xenografts by showing no allergic reactions and no adverse effects emerged.
Results indicated that patients experienced less pain on the Tilapia-treated side when compared to the side treated with paraffin gauze, which proved that Tilapia provided better patient comfort. Tests showed the paraffin gauze group experienced frequent wound fluid leakage, which did not occur with Tilapia skin since it established a beneficial healing condition.
The research demonstrates that Tilapia skin xenografts present more advantages as burn dressings because they help maintain better moisture balance while needing fewer changes and providing better experiences compared to traditional paraffin-based dressings. 43
The available research shows that tilapia skin represents an appropriate tool to address burn healing when conventional skin grafts cannot be employed. Additional studies are required to completely comprehend the possible advantages of tilapia skin applications while developing official standards for its medical use.
Comparison of Commonly used Wound Dressings for Burn Injuries
This comparison of wound dressings for burn injuries shown in Table 1 is based on data from scientific evidence supporting Tilapia skin in burn treatment, where multiple studies have demonstrated the efficacy of Tilapia skin as a burn dressing. To assess its advantages, Tilapia skin is compared to Silver Sulfadiazine and Paraffin Gauze, two widely used standards in burn care. The analysis investigates clinical performance through investigation, including healing time, infection rate, pain management, scar formation, and patient comfort, alongside ease of use and adverse reactions evaluation to demonstrate superior potential in Tilapia skin usage. 48
Table 1: Comparison of Tilapia Fish Skin to Paraffin Gauze and Silver Sulfadiazine
|
Criteria |
Tilapia Fish Skin |
Paraffin Gauze |
Silver Sulfadiazine |
|
Healing Time |
9.77 – 18.10 days (faster epithelialization) |
14-21 days (varies with wound depth) |
18-21 days (due to frequent dressing changes) |
|
Infection Rate |
Lower (natural antibacterial properties) |
Moderate (infection risk varies depending on dressing use) |
Low (due to antibacterial action) |
|
Pain Management |
Better (natural analgesic effect, fewer dressing changes) |
Moderate (requires regular dressing changes) |
Moderate to Poor (frequent changes cause pain) |
|
Scar Formation |
Reduced (collagen-rich skin promotes healing) |
Higher (scarring depends on wound care) |
Higher (due to repeated dressing changes) |
|
Patient Comfort |
High (less frequent dressing changes) |
Moderate |
Low (pain and frequent changes) |
|
Ease of Use |
Moderate (requires specific handling) |
Easy |
Easy |
|
Adverse Reactions |
Minimal (rare allergic reactions) |
Possible (allergy to paraffin) |
Possible (allergic reactions, delayed healing) |
The data indicate that tilapia fish skin functions as an effective solution for treating burn wounds. It offers faster healing times, reduced infection rates, better pain management, and potentially lower scar formation than when using paraffin gauze and silver sulfadiazine. The benefits of using tilapia fish skin outweigh the handling requirements that the product demands. However, additional investigations are needed to determine both the security and effectiveness of tilapia fish skin use in different patient groups during extended treatment periods.
Clinical Advancements and Future Directions
As research progresses, Tilapia skin is being further optimized for clinical applications through advanced formulations:
Tilapia Fish Skin Collagen Extract Ointment
A 15% Tilapia collagen extract ointment has demonstrated superior wound healing in preclinical studies, significantly reducing burn wound size compared to conventional treatments. The extraction method involves advanced purification techniques, including NaOH and butyl alcohol pre-treatment, acetic acid extraction, and NaCl precipitation, followed by dialysis and freeze-drying steps to extract bioactive collagen, which optimizes its skin regeneration effects. The natural biocompatible ointment differs from traditional burn treatments by providing better hydration and better elasticity, along with decreased scarring effects. The ointment satisfies all pharmaceutical requirements, such as homogeneity along with spreadability and pH stability (pH 4-5), which makes it commercializable worldwide for burn care applications. 44
Processed Tilapia Collagen Sponges: Smart Wound Healing with Natural Biomaterials
Collagen sponges derived from Tilapia skin, including Dialyzed Tilapia Skin Collagen Sponge (DTSCS) and Self-Assembled Tilapia Skin Collagen Sponge (STSCS), present a next-generation wound dressing solution, offering improved hemorrhage control and biocompatible healing along with enhanced tissue regeneration properties. The freeze-drying method used to produce these sponges contains no cross-linking chemicals, which results in sponges that exhibit high biocompatibility with less immune response. The sponge structure contains numerous pores, which allow them to sustain favorable wound conditions through effective fluid absorption while speeding up tissue restoration processes. DTSCS and STSCS maintain high levels of porosity (91.25% – 97.38%) that allow them to efficiently absorb water as well as wound exudates, thereby supporting moist healing conditions. Their loose fiber network of collagen serves three wound-healing functions, which include fast cellular movement while promoting tissue regeneration and enhancing nutritional exchange through the network.
The analysis between Tilapia-based sponges showed that STSCS achieved a better outcome compared to conventional bovine collagen sponges, demonstrating faster wound closure, superior biocompatibility, and a reduced inflammatory response by day 14. Scientific investigations demonstrate that STSCS works exceptionally well to stop bleeding in rat models of hepatic and arterial bleeding, within 60 seconds, to stabilize bleeding streams superior to standard dressings and DTSCS. The empirical data suggest Tilapia skin collagen sponges would function as a superior choice to ordinary wound dressing materials by providing three essential features: gradual integration with tissue, accelerated wound restoration, and powerful bleeding arrest capability. These burn care materials are supported by their cost-effectiveness and sustainable production from fish byproducts to create sustainable products. 45
Decellularized Tilapia Skin Scaffolds Enhanced with Silver Nanoparticles: A Dual-Action Burn Therapy
The research has established a decellularized fish skin (DFS) scaffold featuring biosynthesized silver nanoparticles (AgNPs) for improving both burn wound healing and protecting against infections. The DFS scaffold maintains its high Type I collagen content to provide tissue regeneration support, and AgNPs offer potent antibacterial properties that stop burn-related infections from developing. The biosynthesized AgNPs obtained from Aloe vera extract underwent characterization to determine size measurements, crystalline properties, and antimicrobial effectiveness levels. The antibacterial potency of nanoparticles at 6-hour incubation reached 29.1 nm, but nanoparticles at 12-hour incubation grew slightly larger at 35.2 nm. The decellularization process yielded a suitable DFS scaffold with intact collagen structure and mechanical properties appropriate for use as a clinical burn dressing. The material exhibits high moisture retention (81.7%) to support wound healing through optimal moist conditions, which helps promote faster re-epithelialization.
Additionally, the scaffold also exhibited high swelling capacity (102.89%), which enabled it to absorb exudates from wounds and safeguard against bacterial proliferation. The AgNP-loaded DFS scaffold proved effective against the major wound pathogens Staphylococcus aureus and Pseudomonas aeruginosa in an antibacterial assay. Tests confirmed that the bactericidal potency of the containing solution reached 50 µg/ml as its minimum inhibitory concentration (MIC). Also, in cytotoxicity studies, the scaffold demonstrated safe characteristics with fibroblast 3T3 cells because it did not cause any toxic effects and simultaneously enhanced cell growth for wound healing purposes. Its antibacterial efficiency, moisture retention, biodegradability, and wound-healing potential make the AgNP-infused DFS scaffold stands as a cost-effective and eco-friendly substitute for synthetic burn dressings. Its biodegradability eliminates the necessity for frequent dressing adjustments, so it is ideal for clinical burn care, especially when healing extends over a protracted period. 46
Tilapia Collagen-Based Hydrogel
Collagen, a key biomaterial for wound healing, can be sustainably extracted, and the fish scale origin provides a cost-efficient biocompatible alternative to traditional sources. The researchers used the Taguchi method optimization to discover the optimal parameters for extracting collagen from fish scales with Tris-Glycine buffer. The optimal conditions for maximum collagen yield (17.14 ± 0.05 mg/g) required 0.5 M acetic acid, 100 mL acid volume with 120-minute soaking, followed by the addition of 10 mL Tris-Glycine buffer. The extracted Type I collagen demonstrated high purity as well as structural integrity and included significant levels of glycine (20.98%), proline (15.43%), and hydroxyproline (11.51%), which make it suitable for wound healing and tissue regeneration.
Hydrogels derived from Tilapia constitute a significant advance in burn treatment by delivering both cooling properties and moisturization and bioactive wound dressing benefits. Hydrogels function as instant pain blockers while simultaneously decreasing inflammation and blocking bacterial infections, which are essential components for burn injury treatment. Medical scientists created a durable hydrogel by crosslinking Tilapia collagen with hyaluronic acid, which demonstrates enhanced mechanical strength. These hydrogels demonstrate enhanced properties compared to traditional synthetic hydrogels because they originate from natural sources, while providing improved biocompatibility, reduced allergenicity, and better wound healing results. The eco-friendly collagen extraction technique allows better recovery of collagen materials, reducing waste from the seafood industry. Research findings demonstrate that fish scale collagen shows promise for medical treatments of burns and advances the development of advanced clinical biomaterials. 47
Evaluation of Tilapia Skin in Burn Care: Advantages and Drawbacks
Advantages
Accessibility and Cost-Effectiveness
Tilapia fish are inexpensive and widely available, which makes their skin a convenient and affordable option for burn treatment. Tilapia skin, as a commonly available byproduct, helps cut down the costs and problems linked to using different wound dressings. This makes it a particularly cost-effective treatment choice. 27
Bioactive Properties for he:
The skin of tilapia contains hydrated type 3 collagen, which is structurally similar to the human skin. This high collagen content helps dressing to adhere effectively to the wound bed, which results in reduced scarring and minimal side effects.27
Reduced Healing Time and Improved Pain Management
Studies have shown that using tilapia skin on burns provides improved results by reducing healing time. The healing process becomes shorter, because of which means patients experience less pain and require fewer pain medications for treatment. The application of tilapia skin benefits patients because of its natural properties, which reduce discomfort. 27
Dressing-Free Treatment
Unlike traditional bandages and ointments that need to be cleaned and changed frequently, tilapia skin can be left on until the burn heals fully. The patient no longer experiences needless agony and discomfort for the patient.27
Infection Resistance
The antibacterial properties and resistance against infections that occur in Tilapia skin make it an optimal material for treating burn wounds. This advantage sets it apart from other options like pigskin, which lacks similar resistance.27
Preparation-Free and Anesthesia-Free:
Unlike pigskin, which requires both animal fasting and anesthesia during treatment, treatment with tilapia skin does not need animal preparation or anesthesia-free techniques, eliminating extra procedures and associated risks.27
Drawbacks
Odor Sensitivity
Tilapia skin develops a distinct odor as it breaks down over time; this odor might displease both patients and their caregivers. While sterilization reduces the initial microbial load, subsequent breakdown of biological material on the wound can still produce odors that may affect patient comfort and compliance. This is not merely an aesthetic issue; a strong, unpleasant odor can negatively impact a patient’s psychological well-being and comfort during a prolonged recovery period. Professional cleaning methods stand as the main approach to reduce this problem. 27
Sterilization Requirements
Before using fish skin purchased from the market, the skin needs full cleaning and sterilization due to the possibility of muscle tissue attachments. The extra step will extend the duration of treatment procedures. Furthermore, the required sterilization methods, such as glycerolization and gamma irradiation, demand specialized laboratory facilities and protocols. This can present a significant logistical challenge, particularly in low-resource settings where tilapia skin is promoted as most beneficial 27
Adherence Challenges
The adherence of tilapia skin becomes challenging for some burn injury locations, which include areas such as the face, groin, buttocks, neck, genitalia, or axillae. These are often areas of high mobility and complex contours, making it difficult for the relatively stiff, sterilized skin to conform perfectly to the wound bed, potentially leading to graft detachment. This can lead to graft displacement or failure, requiring more frequent monitoring and potentially alternative dressing methods for these specific regions.27
Conclusion
Burn wound treatment using tilapia fish skin introduces a promising and advantageous solution to traditional skin dressing methods, silver sulfadiazine, and paraffin gauze. Its rich composition of collagen, omega-3 fatty acids, and bioactive compounds contributes to accelerated healing, pain reduction, and potentially minimized scarring, while also providing strong infection resistance. The cost-effectiveness and broad availability of tilapia fish skin prove to be a superior choice for burn care, particularly within healthcare settings where resources are limited. However, challenges related to odor sensitivity, sterilization requirements, and adhesive challenges in particular areas are important considerations for clinical implementation. Dressing changes become fewer, and patient discomfort is reduced through these improvements, which enhance both comfort and treatment effectiveness. While more research is needed to optimize processing, establish universal standardization protocols, and improve application techniques, tilapia skin holds immense potential in revolutionizing burn care, offering an affordable, efficient, and biocompatible alternative that can improve wound healing on a global scale. The application shows promising potential to boost present-day burn care strategies, especially in areas without access to traditional medical tools.
Acknowledgment
All the authors are sincerely acknowledge the valuable guidance and support of Dr. S.S. Sonawane during the preparation of this review article. Gratitude is also extended to the Department of Quality Assurance, MET Institute of Pharmacy, Nashik, for providing the necessary facilities and academic environment.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Permission to reproduce material from other sources
Not Applicable
Author Contributions
Harshada Suren Patil – Wrote the final draft of the work and edited
Kaveri Shantaram Panpatil – Completed all reference work and formatting
Shruti Deepak Shinde – Collected all the data and completed the literature review
Priyanka Sanjay Wabale – Assisted in data collection and performed all data editing
Sandeep Sonawane – Supervised the entire work and provided valuable guidance
References
- World Health Organization. Burns. WHO. Published October 13, 2023. https://www.who.int/news-room/fact-sheets/detail/burns
- Smolle C, Cambiaso-Daniel J, Forbes AA, et al. Recent trends in burn epidemiology worldwide: A systematic review. Burns. 2017;43(2):249-257. doi:1016/j.burns.2016.08.013
CrossRef - Barrett LW, Fear VS, Waithman JC, Wood FM, Fear MW. Understanding acute burn injury as a chronic disease. Burns & Trauma. 2019;7(1). doi:1186/s41038-019-0163-2
CrossRef - Lima Júnior EM, De Moraes Filho MO, Costa BA, et al. Innovative Burn Treatment Using Tilapia Skin as a Xenograft: A Phase II Randomized Controlled Trial. Journal of Burn Care & Research: Official Publication of the American Burn Association. 2020;41(3). doi:1093/jbcr/irz205
CrossRef - Alam K, Jeffery SLA. Acellular Fish Skin Grafts for Management of Split-Thickness Donor Sites and Partial-Thickness Burns: A Case Series. Military Medicine. 2019;184(Supplement_1):16-20. doi:1093/milmed/usy280
CrossRef - Baldursson BT, Kjartansson H, Konrádsdóttir F, Gudnason P, Sigurjonsson GF, Lund SH. Healing Rate and Autoimmune Safety of Full-Thickness Wounds Treated With Fish Skin Acellular Dermal Matrix Versus Porcine Small-Intestine Submucosa. The International Journal of Lower Extremity Wounds. 2015;14(1):37-43. doi:1177/1534734615573661
CrossRef - Long V. The Evolution of Burn Therapy—Then and Now. JAMA Dermatology. 2017;153(2):136. doi:1001/jamadermatol.2016.0164
CrossRef - Ozhathil DK, Tay MW, Wolf SE, Branski LK. A Narrative Review of the History of Skin Grafting in Burn Care. Medicina. 2021;57(4):380. doi:3390/medicina57040380
CrossRef - Moiemen NS, Lee KC, Joory K. History of burns: The past, present and the future. Burns & Trauma. 2014;2(4):169. doi:4103/2321-3868.143620
CrossRef - Liu HF, Zhang F, Lineaweaver WC. History and Advancement of Burn Treatments. Annals of Plastic Surgery. 2017;78(2):S2-S8. doi:1097/sap.0000000000000896
CrossRef - Milner S. Classification of Burn Depth. Eplasty. 2024;24:QA5. https://pubmed.ncbi.nlm.nih.gov/ 38501145/
- Szymanski KD, Tannan SC. Thermal Burns. StatPearls Publishing.Nih.gov. Published May 29, 2023. Accessed May 14, 2025. https://www.ncbi.nlm.nih.gov/sites/books/NBK430773/
- Spies C, Trohman RG. Narrative Review: Electrocution and Life-Threatening Electrical Injuries. Annals of Internal Medicine. 2006;145(7):531. doi:7326/0003-4819-145-7-200610030-00011
CrossRef - Koh DH, Lee SG, Kim HC. Incidence and characteristics of chemical burns. Burns. 2017;43(3):654-664. doi:1016/j.burns.2016.08.037
CrossRef - Hettiaratchy S, Dziewulski P. Pathophysiology and types of burns. BMJ. 2004;328(7453):1427-1429. doi:1136/bmj.328.7453.1427
CrossRef - Demling RH. Fluid Replacement in Burned Patients. Surgical Clinics of North America. 1987;67(1):15-30. doi:1016/s0039-6109(16)44130-7
CrossRef - Galeiras R. Smoke inhalation injury: a narrative review. Mediastinum. 2021;5(16):16-16. doi:21037/med-21-7
CrossRef - Panchal A, Casadonte J. Burn‐induced myocardial depression in a pediatric patient leading to fulminant cardiogenic shock and multiorgan failure requiring extracorporeal life support. Clinical Case Reports. 2020;8(4):602-605. doi:1002/ccr3.2667
CrossRef - Burgess M, Valdera F, Varon D, Kankuri E, Nuutila K. The Immune and Regenerative Response to Burn Injury. Cells. 2022;11(19):3073. doi:3390/cells11193073
CrossRef - Allahham A, Rowe G, Stevenson A, Fear MW, Ann-Maree Vallence, Wood FM. The impact of burn injury on the central nervous system. Burns & Trauma. 2024;12. doi:1093/burnst/tkad037
CrossRef - Davies JWL. Prompt cooling of burned areas: a review of benefits and the effector mechanisms. Burns. 1982;9(1):1-6. doi:1016/0305-4179(82)90127-9
CrossRef - Alvarado R, Chung KK, Cancio LC, Wolf SE. Burn resuscitation. Burns. 2009;35(1):4-14. doi:1016/j.burns.2008.03.008
CrossRef - Kim H, Shin S, Han D. Review of the History of Basic Principles of Burn Wound Management. Medicina. 2022;58(3):400. doi:3390/medicina58030400
CrossRef - Yoshino Y, Ohtsuka M, Kawaguchi M, et al. The wound/burn guidelines – 6: Guidelines for the management of burns. The Journal of Dermatology. 2016;43(9):989-1010. doi:1111/1346-8138.13288
CrossRef - Kaur S, Auger C, Jeschke MG. Adipose Tissue Metabolic Function and Dysfunction: Impact of Burn Injury. Frontiers in Cell and Developmental Biology. 2020;8. doi:10.3389/fcell.2020.599576
CrossRef - Radzikowska-Büchner E, Łopuszyńska I, Flieger W, Tobiasz M, Maciejewski R, Flieger J. An Overview of Recent Developments in the Management of Burn Injuries. International Journal of Molecular Sciences. 2023;24(22):16357-16357. doi:3390/ijms242216357
CrossRef - Indhuja R.B., Mahalakshmi A. Use of Tilapia skin for burn treatment in both humans and animals. International Journal of Innovative Research in Technology. 2022;8(8):416-419. Accessed May 14, 2025. doi: ijirt 153724
- Lima Júnior EM, Moraes Filho MO de, Forte AJ, et al. Pediatric Burn Treatment Using Tilapia Skin as a Xenograft for Superficial Partial-Thickness Wounds: A Pilot Study. Journal of Burn Care & Research. 2019;41(2). doi:1093/jbcr/irz149
CrossRef - Song WK, Liu D, Sun LL, Li BF, Hou H. Physicochemical and Biocompatibility Properties of Type I Collagen from the Skin of Nile Tilapia (Oreochromis Niloticus) for Biomedical Applications. Marine Drugs. 2019;17(3):137. doi:3390/md17030137
CrossRef - Paula A, Elisa M, Carlos AE, et al. Avaliação microscópica, estudo histoquímico e análise de propriedades tensiométricas da pele de tilápia do Nilo. Rev bras queimaduras. Published online 2015:203-210. https://pesquisa.bvsalud.org/portal/resource/pt/biblio-1402176
- Riyadi PH, Suprayitno E, Aulanni’am A, Sulistiyati TD. Chemical Characteristics and Amino Acids Profile of Protein Hydrolysates of Nile Tilapia (Oreochromis niloticus) Viscera. Journal of World’s Poultry Research. 2019;9(4):324-328. doi:36380/scil.2019.wvj41
CrossRef - Lima Júnior EM, de Moraes Filho MO, Costa BA, et al. Nile Tilapia Fish Skin–Based Wound Dressing Improves Pain and Treatment-Related Costs of Superficial Partial-Thickness Burns: A Phase III Randomized Controlled Trial. Plastic & Reconstructive Surgery. 2021;147(5):1189-1198. doi:1097/prs.0000000000007895
CrossRef - Zimba BL, Rwiza MJ, Sauli E. Utilizing Tilapia Fish Skin Biomaterial for Burn Wound dressing: a Systematic Review. Scientific African. 2024;24:e02245. doi:1016/j.sciaf.2024.e02245
CrossRef - Lima Júnior EM, De Moraes Filho MO, Costa BA, et al. Innovative Burn Treatment Using Tilapia Skin as a Xenograft: A Phase II Randomized Controlled Trial. Journal of Burn Care & Research: Official Publication of the American Burn Association. 2020;41(3). doi:1093/jbcr/irz205
CrossRef - Alves APNN, Lima Júnior EM, Piccolo NS, et al. Study of tensiometric properties, microbiological and collagen content in Nile tilapia skin submitted to different sterilization methods. Cell and Tissue Banking. 2018;19(3):373-382. doi:1007/s10561-017-9681-y
CrossRef - Lima Verde MEQ, Ferreira-Júnior AEC, de Barros-Silva PG, et al. Nile tilapia skin (Oreochromis niloticus) for burn treatment: ultrastructural analysis and quantitative assessment of collagen. Acta Histochemica. 2021;123(6):151762. doi:1016/j.acthis.2021.151762
CrossRef - Michael S, Winters C, Khan M. Acellular Fish Skin Graft Use for Diabetic Lower Extremity Wound Healing: a Retrospective Study of 58 Ulcerations and a Literature Review.Wounds. 2019;31(10):262-268. PMID: 31730505
- Aldonza G, Paulina Nieto Villaseñor, Yaneli A, Rodriguez D. The Use of Fish Skin (Tilapia) in Burn Patients as a New Therapy Under Study. International journal of medical science and clinical research studies. 2023;03(05). doi:47191/ijmscrs/v3-i5-09
CrossRef - Le Guellec D, Morvan-Dubois G, Sire JY. Skin development in bony fish with particular emphasis on collagen deposition in the dermis of the zebrafish (Danio rerio). The International Journal of Developmental Biology. 2004;48(2-3):217-231. doi:1387/ijdb.15272388
CrossRef - Brown P. Regular review: Bovine Spongiform Encephalopathy and Variant Creutzfeldt-Jakob Disease. BMJ. 2001;322(7290):841-844. doi:1136/bmj.322.7290.841
CrossRef - Kjartansson H, Olafsson IH, Karason S, et al. Use of Acellular Fish Skin for Dura Repair in an Ovine Model: A Pilot Study. Open Journal of Modern Neurosurgery. 2015;05(04):124-136. doi:4236/ojmn.2015.54021
CrossRef - Lima-Junior EM, de Moraes Filho MO, Costa BA, et al. Innovative treatment using tilapia skin as a xenograft for partial thickness burns after a gunpowder explosion. Journal of Surgical Case Reports. 2019;2019(6). doi:1093/jscr/rjz181
CrossRef - Putri NM, Kreshanti P, Syarif AN, Duhita GA, Johanna N, Wardhana A. Efficacy of tilapia skin xenograft compared to paraffin-impregnated gauze as a full-thickness burn dressing after excisional debridement: A case series. International Journal of Surgery Case Reports. 2022;95:107240. doi:1016/j.ijscr.2022.107240
CrossRef - Romy Triadi Nugroho, Gilang Saputra, Annisa Nurul Aini, Andini A, Indra Lasmana Tarigan. Ointment Formulation from Collagen Extract of Tilapia Fish Skin (Oreochromis niloticus) for Healing Burns in Mus musculus. Pharmaceutical Journal of Indonesia. 2022;8(1):9-15. doi:21776/ub.pji.2022.008.01.2
CrossRef - Wang T, Yang L, Wang G, et al. Biocompatibility, hemostatic properties, and wound healing evaluation of tilapia skin collagen sponges. Journal of Bioactive and Compatible Polymers. 2020;36(1):44-58. doi:1177/0883911520981705
CrossRef - Adhikari SP, Paudel A, Sharma A, et al. Development of Decellularized Fish Skin Scaffold Decorated with Biosynthesized Silver Nanoparticles for Accelerated Burn Wound Healing. Dunne N, ed. International Journal of Biomaterials. 2023;2023:1-18. doi:1155/2023/8541621
CrossRef - Menezes M do LLR, Ribeiro HL, Abreu F de OM da S, Feitosa JP de A, Filho M de SM de S. Optimization of the collagen extraction from Nile tilapia skin (Oreochromis niloticus) and its hydrogel with hyaluronic acid. Colloids and Surfaces B: Biointerfaces. 2020;189:110852. doi:1016/j.colsurfb.2020.110852
CrossRef - Moraes FCA de, Ferraz Barbosa B, Sepulvida D, et al. Nile Tilapia Skin Xenograft Versus Silver-Based Dressings in the Management of Partial-Thickness Burn Wounds: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2024;13(6):1642. doi:3390/jcm13061642
CrossRef
Abbreviations
ARDS: Adult Respiratory Distress Syndrome;
AgNPs: silver nanoparticles; DFS, Decellularized Fish Skin;
DTSCS: Dialyzed Tilapia Skin Collagen Sponge;
MIC: Minimum Inhibitory Concentration;
PTSD: Post-Traumatic Stress Disorder;
RCT: Randomized Controlled Trial;
SSDC: Silver Sulfadiazine Cream;
STSCS: Self-Assembled Tilapia Skin Collagen Sponge;
TBSA: Total Body Surface Area;
TNF: Tumor Necrosis Factor;
VAS: Visual Analogue Scale;
BC: Before Christ.

This work is licensed under a Creative Commons Attribution 4.0 International License.





