Manuscript accepted on : 08-05-2025
Published online on: 26-05-2025
Plagiarism Check: Yes
Reviewed by: Dr. Thanaa Naji Shaker Abuguname![]()
Second Review by: Dr. Marwa Husam
Final Approval by: Dr. Jagdish Chandra Joshi
Evaluating Nanotoxicity: Integrating Invitro and Invivo Models for Risk Assessment
Vasantha Galanki , Mohini Rangala*
, Mohini Rangala* , Iragavarapu Tejolahari
, Iragavarapu Tejolahari , Gajula Niharika
, Gajula Niharika and Allampalli Likhita
and Allampalli Likhita
Department of Pharmacology, Vignan Institute of Pharmaceutical Technology, Visakhapatnam, India
Corresponding Author E-mail: rangalamohini968@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3374
ABSTRACT: Nanotechnology, involving the manipulation of materials at the nanoscale, has vast applications in healthcare, including drug delivery, gene therapy, and cancer treatment. Nanomaterials (NM) such as nanoparticles (NP’s) and nanotubes exhibit unique properties that raise concerns about potential toxicity. Nanotoxicology studies the safety of these materials, focusing on their physicochemical properties and toxicological effects like genotoxicity and cellular damage. In vitro methods, including cytotoxicity, apoptosis, and genotoxicity assays, provide controlled environments for testing nanoparticle interactions with biological systems. In vivo methods further assess the effects in living organisms, including biodistribution and histopathological changes. New technologies, such as high-throughput screening, Nano-QSAR (quantitative structure -activity relationship) computational models, and stem cell-based assays, are enhancing toxicity prediction. Omics technologies (e.g., genomics and proteomics) offer a comprehensive understanding of how nanoparticles affect biological systems. These advanced techniques are crucial for ensuring the safe development and use of nanomaterials.
KEYWORDS: Genotoxicity; Invitro methods; Nanotoxicology; Nanoparticles; Nanotechnology
Download this article as:| Copy the following to cite this article: Galanki V, Rangala M, Tejolahari I, Niharika G, Likhita A. Evaluating Nanotoxicity: Integrating Invitro and Invivo Models for Risk Assessment. Biotech Res Asia 2025;22(2). |
| Copy the following to cite this URL: Galanki V, Rangala M, Tejolahari I, Niharika G, Likhita A. Evaluating Nanotoxicity: Integrating Invitro and Invivo Models for Risk Assessment. Biotech Res Asia 2025;22(2). Available from: https://bit.ly/3FoC07C |
Introduction
The prefix “nano” originates from the Greek word “nanos,” which means “dwarf.” Nanotechnology is the manipulation and application of artificial particles or systems that have at least one dimension smaller than 100 nanometers (nm). A structure that has at least one size (length, height, and breadth) smaller than 100 nanometers (10-7 meters) and as huge as a virus particle is designated as a nanomaterial. They are categorized depending on their size, dimensions (0, 1, 2, 3D), composition, shape (nanoparticle, nanotube, nanostick, nanofiber etc.), source (natural, synthetic), and content (carbon-derived, composite-based organic-based, etc.). The most often used types of nanomaterials are nanoparticles, which have three dimensions that are all equal and smaller than 100 nanometers.1 “Nanoparticles” that are found naturally or as results of other operations, such as carbon black, fire smoke, or welding fumes; rather, the word refers solely to manmade particles (such as nanotubes made of carbon, iron oxides, fullerenes, etc.2 Humans are exposed to nanoparticles both directly and indirectly as a result of their growing use in commercial applications such as semiconductor components, skincare products, water filtration, fillers, opacifiers, catalysts, and microelectronics.3 In order to target certain cells, biomolecules like DNA, proteins, and monoclonal antibodies are frequently purposefully coated onto nanomaterials for imaging and drug delivery.4When it comes to their use in commercial items, industrial processes, and biology, nanomaterials have had a significant impact. This technology, known as nanotechnology, has the potential to bring about a new revolution in society.5
Therapeutic applications of Nanoparticles a relatively recent subject of the health sciences, is one of the emerging fields. Consequently, a number of nanoparticles are being investigated or employed in several clinical domains, such as therapy, smart ways to deliver drugs, surgery, medical implants, treatment of illnesses or cancers, and gene delivery as mentioned in the Figure 1.
Gene therapy
The intention of gene therapy, a subject that is extensively researched, is to fix malfunctioning genes in order to prevent and treat genetic illnesses.
Antibacterial activity
Multiple silver nanoparticles have been demonstrated to possess antibacterial activity; these can also be used alongside with other drugs for reducing antibiotic resistance.
ZnO(Zinc oxide) nanoparticles may be regarded as a useful adjuvant in ciprofloxacin combination therapy due to their possible synergistic action with the antibiotic.6
Cancer diagnosis and treatment
Carbon nanotubes are employed in the identification of biomarkers and the disclosure of DNA alterations.7 Quantum dots are used with magnetic resonance imaging to more precisely reveal tumor location.8
Neurological impairment therapy
Applications of nanotechnologies show promise in the management of the neurological disorder and the repair of damaged axons. Utilizing nanoparticles with a strong affinity for circulating amyloid-β with an emphasis on protecting neuronal tissue.9
Orthopedic implants
Collagen, hyaluronic acid, chitosan, and titanium alloys are examples of natural and synthetic polymers that are frequently utilized as nanomaterials in bone and cartilage tissue engineering.10
Therapies for skin health
Novel nanoscale materials that have been created and produced to solve current wound care issues. Drug delivery, growth factor supplements, hydrogels, biodendrimers, electrospun nanofibers, and other polymer therapeutic conjugates have all been employed as skin substitutes in wound healing procedures.11
Nanotoxicity
A novel area of toxicology called nanotoxicology was suggested to fill in the information gaps and to explicitly address the potential negative health effects of nanomaterials. Nanomaterials are being used more and more in industrial applications, consumer goods, and medical devices as nanotechnology develops quickly. Their distinct characteristics, like size, surface area, and reactivity, can, nevertheless, result in unanticipated toxicological effects that are not found with bulk materials. Nanotoxicology encompasses the domains of exposure pathways, molecular determinants, physicochemical factors, biodistribution & genotoxicity.12
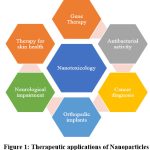 |
Figure 1: Therapeutic applications of Nanoparticles Click here to view Figure |
Physicochemical properties of nanoparticles
Nanoparticles possess unique physicochemical properties that distinguish them from their bulk counterparts, such as extremely small size and a high surface area-to-volume ratio as shown in the Figure 2. These features grant them enhanced reactivity, improved cellular uptake. However, the same properties that provide functional advantages can also introduce novel mechanisms of toxicity. Their small size allows nanoparticles to interact with cellular structures at the molecular level, potentially disrupting normal biological functions. High surface reactivity can lead to the generation of reactive oxygen species (ROS), causing oxidative stress, inflammation, and cellular damage. Different shapes and morphologies, such as rods or fibers, may lead to mechanical damage or hinder cellular clearance, prolonging exposure. Thus, nanoparticle-induced toxicity arises not from a single factor but from a complex interplay of size, surface area, composition, shape, and other physicochemical traits.
Particle size and surface area
Materials’ surface area seems to grow exponentially in proportion to their volume as they get smaller, which raises the nanomaterial’s surface reactivity to its surroundings and to itself. Notably, particle size and surface area affect how the system responds to, distributes, and eliminates the materials.14 To evaluate the in vitro cytotoxicity of NPs of different sizes, several groups have employed a variety of cell types, incubation conditions, and duration of exposure.15,16 One of the primary reasons for the toxicity of ENMs(Engineered Nanomaterials) in vivo is the generation of reactive oxygen species through the creation of free radicals. Dimensions has significance in this process because, as many writers have observed, the smaller the object, the more likely it is to generate ROS(Reactive oxygen species).17 Numerous investigations utilizing a range of nanoparticle classes demonstrated that surface area plays a key role in exhibiting hazardous effects.18 While NPs larger than 50 nm do not spread to other tissues and are readily absorbed by RES(Reticulo endothelial system), NPs smaller than 50 nm have been found to traverse rapidly to almost all tissues and produce possibly harmful symptoms in a variety of tissues.19
Effect of Particle Shape
Numerous nanoparticles, such as silica, allotropies, carbon nanotubes, gold, nickel and titanium nanomaterials have been shown to have shape-dependent toxicity.20-23 In contrast to nanoparticles that are fibre-like or rod-shaped nanoparticles, spherical nanoparticles have been found to undergo endocytosis more easily and quickly.24Likewise, was demonstrated that the absorption of gold nanorods is not as quick as spherical nanospheres and that it peaks when the aspect ratio is close to unity.25
Effect of Surface Charge
Surface charge has a substantial impact on nanoparticle toxicity as well. Because of their improved opsonization by the plasma proteins, positively charged nanoparticles exhibit notable cellular absorption in contrast to negatively charged and neutral nanoparticles. Additionally, they have been demonstrated to cause platelet aggregation and hemolysis,26 besides it was also noted that the surface charge of nanoparticles modifies the transmembrane permeability and blood-brain barrier integrity.
Effect of Aggregation and Concentration
Research findings demonstrated that well-dispersed carbon nanotubes had cytotoxic effects when contrasted with those of conventionally purified rope-like agglomerated carbon nanotubes and asbestos as a reference.27
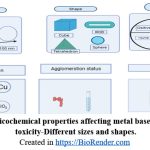 |
Figure 2: Physicochemical properties affecting metal based nanoparticle toxicity-Different sizes and shapes.Click here to view Figure |
Created in https://BioRender.com
Routes of exposures
One definition of “exposure” is the potential for an agent or substance from the outside environment, such as a nanoparticle (NP), to enter the body as shown in the Figure 3. Furthermore, the potential toxicity is determined by the exposure route.28
Inhalation Exposures
Exposures through inhalation are known to occur largely in professional environments, have been the focus of a large portion of exposure and toxicological research efforts and measurements using NPs to date. In fact, according to research on animals, the majority of the harmful effects of inhaled NPs or particles affect the cellular components in the anatomical compartment of the respiratory tract, namely at the portal entrance sites. The lungs capillaries are where carbon dioxide and oxygen exchange gases. Although the possibility is thought to be minimal or infrequent, it is possible that inhaled NPs could eventually enter the systemic circulation through this pathway.29
Dermal Exposure
In essence, the skin is made up of two main sections: the larger, known as the epidermis, and the dermal component, which is located underneath the epidermis and has a restricted supply of blood vessels. Even after prolonged application, Lademann noted that no particles were seen in the stratum corneum’s deeper levels.30 Exposures via the gastrointestinal tract happen after consuming food or after swallowing and pulmonary clearance of inhaled nanoparticles.31
Oral or Ingestion Exposures
More precisely, food coloring, supplements, and flavor enhancers are a few examples of ingested component sources.32 Depending on the kind of nanoparticle and its characteristics, the possibility that it will pass through the digestive system varies; many are simply expelled without entering the body. According to some researchers, oral exposure to NPs may cause absorption through the gut-associated lymphoid tissue’s Peyer’s patches’ epithelial cells. On an average, micro-copper particles do not cause significant harm to the liver, kidney or spleen of experimental mice but nanoparticles do. The findings point to a gender-dependent aspect of nanotoxicity. The findings point to a gender-dependent aspect of nanotoxicity. 33 According to recent research,34 NPs administered orally may be absorbed through the lymph nodes and travel throughout the gastrointestinal tract.
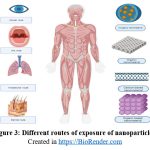 |
Figure 3: Different routes of exposure of nanoparticles.Click here to view Figure |
Created in https://BioRender.com
Biodistribution of Nanoparticles
Physical clearance processes (such as epithelial endocytosis, mucociliary movement, lymphatic drainage, interstitial translocation& blood circulation,) and chemical clearance processes (such as leaching, protein binding & dissolution) are implicated in the studies conducted thus far. Certain nanoparticles may build up in the liver as a result of first-pass metabolism.35 Nanoparticles are transferred to the bone marrow, colon, lungs, spleen, liver and lymphatics following intravenous injection.36 Differences in rate of clearance & confinement of nanoparticles by macrophages are a result of differential opsonization.37 Opsonization events must be suppressed at certain locations or anatomical compartments in order to enhance the non-invasive retention of nanoparticles in the bloodstream. For instance, a poly(ethylene) glycol (PEG) coating would make hydrophobic particles more hydrophilic, lengthening the time it takes for them to circulate throughout the body.38
Genotoxicity
The genotoxic potential of NM exposure is a major worry. The ability of physical or chemical substances to change genetic information is known as genotoxicity. Genotoxic events can result in permanent alterations (mutations) in the quantity or structure of a cell’s genetic material, or they might be temporary (repairable harm). A genetic condition may result from a mutation in a germ cell that is passed down to the following generation. Cancer in somatic cells can result from a mutation in a crucial gene. Therefore, all mutagens are thought to have the potential to cause cancer.39 Several studies have been conducted on the genotoxicity of NMs and the processes that result in temporary or permanent genetic alterations. According to recent research, there are two primary (direct or indirect) and secondary genotoxicity mechanisms that might cause NM genotoxicity.40,41Physical contact between NMs and DNA in the nucleus is necessary for primary direct genotoxicity, which is brought on by direct interaction between NMs and the genome. Large DNA malformations, chromosomal damage, and DNA breaks and other diseases could result from this interaction. Physical contact between NMs and DNA in the nucleus is necessary for primary direct genotoxicity, which is brought on by direct interaction between NMs and the genome. Large DNA malformations, chromosomal damage, and DNA breaks and other diseases could result from this interaction. The main indirect mechanism is caused by reactive oxygen species (ROS) triggered by NMs or by toxic ions released during NM dissolution and intracellular ROS formation through the Fenton-type reaction.42,43 The primary mechanism of NM genotoxicity is thought to be secondary genotoxicity. ROS generated by inflammatory cells mediate it. By activating phagocytes, NMs can generate an oxidative burst. This inflammation, which aids in clearance, is the body’s first line of defense against the invasion of microbes or other objects.44A range of tests covering DNA damage, gene mutations, and chromosomal damage as sensitive genotoxicity endpoints are included in the category of genotoxicity biomarkers. The number of NMs produced is constantly increasing despite the lack of information and limited data regarding NM safety. For this reason, attempts have been made to comprehend how NMs work and to create or modify approved OECD testing procedures for chemicals for use on NMs in a number of previous and ongoing European projects, including NanoGENOTOX, NanoTEST, NanoReg, RiskGONE, HISENTS, RiskGONE, and others.45 There are issues with the mode of action (MoA), specificity and data of regulatory In vitro genotoxicity testing.46
Methods for assessing toxicity of nanomaterials
In Vitro Methods
They are essential to nanotoxicology because they offer a controlled setting for examining interactions of nanoparticles with biological systems, including human cells, tissues, and other model species. In vitro toxicity studies often look at the following parameters: oxidative stress, inflammatory alterations, apoptosis, DNA mutation/damage and cytotoxicity. Inflammatory alterations are an additional parameter that can be assessed using in vitro testing. Inflammatory indicators can be found using the enzyme-linked immunosorbent test (ELISA).47Moreover, in vitro tests can also be used to assess cytotoxicity or necrosis. Assays for cytotoxicity include lactate dehydrogenase (LDH), clonogenic assays, Trypan blue assays, Tetrazolium salts assays, and Alamar blue assays.48
Proliferation Assay
One important factor in the growth and progression of tumors is cell proliferation, which can also represent the surrounding cells’ compensating reaction to necrosis or apoptosis. Currently, there are several well-established techniques for measuring cell proliferation each with unique limitations and specificity. Currently used techniques include flow cytometry, immunohistochemistry, and histochemistry. Histochemical techniques include the absorption of the DNA analog bromodeoxyuridine (BrdU), incorporation of tritiated thymidine ([3H] thymidine), and direct detection of mitosis by staining for DNA content48. In immunohistochemistry, Ki-67 and antibodies that identify proliferating cell nuclear antigens (PCNAs) are now of interest.49
Apoptosis Assay
This assay assays the activity of caspase-3 and caspase-7 using luminescence. Cell lysis and substrate cleavage by caspase occur when caspase reagent is added to NP-treated cells. Luciferase then generates a luminous signal that is equivalent to the amount of caspase activity that is available.50
Genotoxicity Assays
Chromosomal aberration induction
This method involves assessing how NPs affect the quantity of cells and alterations in the physical appearance of chromosomes. Cells are treated with NPs during the S-phase because they are more responsive during this phase. Colcemid or colchicine is then administered at predetermined intervals. Trypsinated cells are counted after chromosomes are processed and stained with Giemsa stain for microscopic evaluation of aberration (chromatid exchange, chromatid breaks, chromosomal breaks, chromosome fragmentation)51 It is employed to measure and evaluate cellular DNA damage. After cells have been embedded in a thin agarose gel and put on a microscope slide, the cells are lysed to extract the cellular proteins. In neutral or alkaline circumstances, allowing the DNA to uncoil before being subjected to electrophoresis, which allows the pieces of DNA to detach from the nucleus. The amount of fluorescence in the head and tail length is determined by staining with either propidium iodide or ethidium bromide. The amount of damaged DNA is directly equivalent to the amount of DNA released from the comet’s head.52,53
Measurement of oxidized guanine bases
Single base changes within a specific gene can be detected by assaying any one of the many oxidized guanine bases, including 7,8-dihydro-oxodeoxyguanine (oxo-dG) and 8-hydroxydeoxyguanosine (8-OHdG). These bases alterations are often caused by oxidative damage and are quantified by HPLC or immunohistochemistry.54
In Vivo Methods
Using animal models, such as mice and rats. In situ toxicity is typically assessed. The methods used to evaluate in vivo toxicity include histopathology, biological distribution, clearance, hemophilia, and plasma chemistry. Biodistribution studies examine how nanoparticles enter organs or tissues. Nanoparticles can be detected in living and expired organisms using radiolabels.55 The liver, kidneys, heart, brain and spleen are among the tissues exposed to nanoparticles, according to histopathology research.56 Examining alterations in cell type and serum chemistry following nanoparticle exposure is an additional method of determining in vivo toxicity.57
Table 1: An overview of the research on nanomaterial toxicity tests
| Test Method | Purpose | Nanomaterial Tested | Outcomes | Year | References |
| Light Microscopy | characteristics of physicochemistry | Singled walled carbon nanotubes, silver particles | Observes the morphology and size of nanomaterials, assessing their properties. | 2006 | 58, 59 |
| Hemoglobin Estimation | Haemolysis | SiO2 | Assesses red blood cell lysis due to exposure to nanomaterials | 2011 | 60 |
| Comet Assay | DNA damage | Metal, Metal oxide nanoparticles | Quantifies the amount of DNA damage (strand breaks) in cells that have been exposed to nanoparticles. | 2010 | 61 |
| Micronucleus test | Genotoxicity | Different types of nanoparticles | Detects DNA damage and chromosomal aberrations caused by nanoparticles. | 2011 | 62 |
| Lactate Dehydrogenase (LDH) Assay | Cell viability | Carbon nanoparticles | Assesses cell membrane integrity and cytotoxicity. | 2005 | 63, 64 |
| Tetrazolium Salts (MTT, MTS, etc.) Assay | Cell viability | Carbon nanoparticles, Fullerenes | Measures metabolic activity of cells to assess cytotoxicity. | 2005;2004 | 65, 66 |
| Alamar Blue Assay | Cell viability | Quantum dots | Detects metabolic activity in cells to evaluate potential toxicity. | 2006 | 67 |
| Caspase-3 Activity Assay | Apoptosis | Silver Nanoparticles | Measures apoptotic cell death by detecting activated caspase-3. | 2009 | 58,59 |
| Lipid Peroxidation, Vitamin E Assay | Oxidative stress | Single-walled carbon nanotubes | Measures lipid peroxidation & antioxidant defense in cells. | 2012 | 68 |
| Reactive Oxygen Species (ROS) Production | Oxidative stress | Oxidative stress | Measures oxidative stress by quantifying ROS production in cells. | 2011 | 69 |
Novel Technologies Employed for Evaluation of Toxicity
Ever since the development of nanotechnology, a growing number of chemicals have entered the environment, necessitating the collection of data regarding their toxicity. Traditional toxicity assessment using animal models is often not practical in these circumstances due to its poor capacity, time-consuming nature, high expense, and limited evaluation of endpoints. The authors claim that this method, in conjunction with functional toxicogenomics, can be crucial in determining the key biological elements and pathways implicated in the toxicity response.70
Genome arrays have been employed to evaluate the impact of nanoparticles. The expression of many genes linked to neurodegenerative illness, immune cell function and motor neuron disorders was altered by the inhaled silver nanoparticles, suggesting possible immunotoxicity and neurotoxicity.71The use of high-throughput screening (HTS) techniques to check how silver nanoparticles affect bacterial cells is said to aid in tracking the ecological consequences of nanoparticles.72 Additionally, HTS might make it possible to create models that forecast how nanoparticles will behave in biological systems. HTS’s objective is to support the safe development of nanomaterials by using quick, automated screening techniques to produce comprehensive, comparable toxicity data for thousands of distinct nanomaterials.73
Computational Models
Computational methods made possible by the development of AI and ML allow nanomedicine to investigate safety issues effectively and economically. The safety assessment at the early phases of drug research, which integrates data at several levels and produces reliable conclusions, has a detrimental impact on the medication’s failure in the drug development process. Understanding the limitations, science and opportunities that underpin this application is crucial to getting the most out of it. Additionally, computational approaches consider the pharmacokinetic elements in addition to the ligand-receptor docking concept in order to illustrate the results.74
Quantitative Structure-Activity Relationship (QSAR) Modeling
Routine nanotoxicity investigations use less time, money, and resources thanks to computational technologies like nano-QSAR models QSAR and (at the nanoscale). Pharmacokinetic and pharmacodynamic data are primarily correlated with in vivo application scenarios using these models.74 QSAR methods use a compound’s physicochemical characteristics to predict its biological activity (75). Later, in 1988, Cramer and associates created a different method known as 3D-QSAR, which takes into account the interactions, activity& spatial structure of molecules.75 One-dimensional (1D), two-dimensional (2D), and three-dimensional (3D) techniques are all covered by Nano-QSAR, making it a very universal model76. The low-energy conformations that docked into the ADME model were used to build the 3D nano-QSAR. Atoms in a crystal are more likely to overlap their orbital energy and divide if they are close to one another. Conduction band overlap suggests that NMs are cytotoxic or have other disruptive effects.76
Stem Cells as a Novel Approach to Predict Toxicity
All of the biological cell can be developed from pluripotent stem cells. The majority of the precise NMs toxicity is still unknown because of the use of imprecise, oversimplified techniques, such as 2D cell lines, in compound testing and validation. Recent stem cell research has focused more on ESCs and iPSCs.77 ESC is produced from an embryo’s undifferentiated inner mass cells, whereas iPSCs are derived from somatic cells that have been reprogrammed to function as ESCs by activating genes or forcing the expression of reprogramming genes such Klf4, Oct4 and Sox2.78
Omics Technologies
“As a whole” is what the suffix “omics” stands for, and it encompasses proteomics, metabolomics, transcriptomics, epigenomics, and genomics. Adaptive responses can be identified using these techniques. Systems toxicology encompasses transcriptomics, proteomics, metabolomics, genomics, and epigenomics (miRNomics and DNA alterations). Using recombinant DNA, DNA sequencing, and bioinformatics to examine the structure and function of the genome, genomics studies genes and their roles. These technologies’ main benefits include the ability to forecast toxicity at low exposure levels to nanoparticles, which can stress cells without causing toxicity.79 NPs cause this pattern and affect a number of biological functions, including membrane integrity, inflammation, apoptosis, and proliferation.80
Conclusion
Nanotoxicology is a rapidly expanding field focused on assessing the risks of nanomaterials used in industries like healthcare and consumer products. While nanotechnology offers significant therapeutic potential, such as in drug delivery and cancer treatment, the unique properties of nanoparticles—such as size, surface area, shape, and charge—can lead to unexpected toxicological effects. Understanding the mechanisms of nanoparticle toxicity, including genotoxicity, inflammation, and oxidative stress, is critical for ensuring their safe use. The biodistribution, clearance, and exposure routes of nanoparticles (inhalation, dermal contact, and ingestion) significantly affect their health impacts. Despite progress, many gaps remain in understanding nanomaterial safety, and more research is needed to establish comprehensive safety standards. Advancements in novel testing methods, including high-throughput screening, computational models, and stem cell-based assays, are essential to assess the long-term effects of nanoparticles and guide their safe development. Both in vitro and in vivo methods are crucial for evaluating the biological effects of nanoparticles, with assays focused on cytotoxicity, oxidative stress, genotoxicity, and apoptosis being commonly used. Emerging technologies like high-throughput screening, computational models, and omics technologies offer efficient, cost-effective ways to predict nanoparticle behaviour and toxicity. These tools enhance our ability to assess the molecular impacts of nanomaterials, identify adaptive responses, and predict low-exposure toxicity. As nanomaterials continue to evolve, integrating these advanced methods is vital for ensuring the safe development and regulation of nanomaterials, protecting both human health and the environment.
Acknowledgement
The Author would like to Profoundly Grateful to the Principal and Management of Vignan Institute of Pharmaceutical Technology (Autonomous). Visakhapatnam.
Funding Sources
The author(s) received no financial support for the research, authorship, and/or publication
of this article.
Conflict of Interest
The authors do not have any conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
This research did not involve human participants, animal subjects, or any material that
requires ethical approval.
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not
required.
Clinical Trial Registration
This research does not involve any clinical trials.
Permission to reproduce material from other sources
Not Applicable
Author Contributions
Galanki Vasantha : Conceptualization, Supervision, Methodology, Writing- Review & Editing.
Rangala Mohini: Writing – Original Draft, Data Collection, Analysis
Iragavarapu Tejolahari: Analysis and Data Collection,
Gajula Niharika: Data Collection and Analysis
Allampalli Likhita: Data Collection and Analysis
Rfferences
- Vance ME, Kuiken T, Vejerano EP, et al. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein Nanotechnol. 2015; 6(1):1769-80. doi: 10.3762/bjnano.6.181
CrossRef - Hoyt VW, Mason E. Nanotechnology: emerging health issues. J. Chem. Health, 2008 ;15(2):10-5.
CrossRef
CrossRef - Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. 2006; 311(5761):622-7. doi: 10.1126/science.1114397
CrossRef
CrossRef - Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. 2008; 4(1):26-49. doi: 10.1002/smll.200700595
CrossRef - Deepa Parvathi V, Rajagopal K. Nanotoxicology testing: Potential of Drosophila in toxicity assessment of nanomaterials. J. Nanosci. Nanotechnol. 2014;5(1):25-35.
- Banoee M, Seif S, Nazari ZE, et al. ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. Biomed. Mater. Res.B Appl Biomater. 2010 ;93(2):557-61. doi: 10.1002/jbm.b.31615
CrossRef - Nahar M, Dutta T, Murugesan S, et al. Functional polymeric nanoparticles: an efficient and promising tool for active delivery of bioactives. Rev. Ther. Drug Carr. Syst. 2006;23(4) :259-318. doi : 10.1615/critrevtherdrugcarriersyst.v23.i4.10
CrossRef - Zheng G, Patolsky F, Cui Y, Wang WU, Lieber CM. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat Biotechnol. 2005 ;23(10):1294-301. doi : 1038/nbt1138
CrossRef - Brambilla D, Le Droumaguet B, Nicolas J, et al. Nanotechnologies for Alzheimer’s disease: diagnosis, therapy, and safety issues. 2011 ;7(5):521-40. doi: 10.1016/j.nano.2011.03.008
CrossRef - Tamjid E, Bagheri R, Vossoughi M, Simchi A. Effect of TiO2 morphology on in vitro bioactivity of polycaprolactone/TiO2 nanocomposites. Materials Letters. 2011;65(15-16):2530-3.
CrossRef - V Singh A, AS A, N Gade W, et alm. Nanomaterials: New generation therapeutics in wound healing and tissue repair. Nanosci. 2010 ;6(6):577-86.
CrossRef - Donaldson K, Stone V, Tran CL, Kreyling W, Borm PJ. Nanotoxicology. Occup Environ Med. 2004 ;61(9):727-8.
CrossRef - Gatoo MA, Naseem S, Arfat MY, et al. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res. 2014;2014(1):498420. doi: 10.1155/2014/498420
CrossRef - Powers KW, Palazuelos M, Moudgil BM, Roberts SM. Characterization of the size, shape, and state of dispersion of nanoparticles for toxicological studies. Nanotoxicology. 2007 ;1(1):42-51. doi:1080/17435390701314902
CrossRef - Yin H, Too HP, Chow GM. The effects of particle size and surface coating on the cytotoxicity of nickel ferrite. Biomaterials. 2005;26(29):5818-26.doi: 1016/j.biomaterials.2005.02.036
CrossRef - Hu Y, Xie J, Tong YW, Wang CH. Effect of PEG conformation and particle size on the cellular uptake efficiency of nanoparticles with the HepG2 cells. Controlled Release. 2007 ;118(1): 7-17. doi: 10.1016/j.jconrel.2006.11.028
CrossRef - Gatoo MA, Naseem S, Arfat MY, Mahmood Dar A, Qasim K, Zubair S. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res.Int.2014;2014(1):498420.
CrossRef - Holgate ST. Exposure, uptake, distribution and toxicity of nanomaterials in humans. Biomed. Nanotechnol., 2010 ;6(1): 1-9. doi : 10.1166/jbn.2010.1098
CrossRef - De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. 2008 ;29(12):1912-9. doi : 10.1016/j.biomaterials.2007.12.037
CrossRef - Petersen EJ, Nelson BC. Mechanisms and measurements of nanomaterial-induced oxidative damage to DNA. Anal and Bioanal Chem. 2010; 398(2) :613-50. doi: 1007/s00216-010-3881-7
CrossRef - Ispas C, Andreescu D, Patel A, Goia DV, Andreescu S, Wallace KN. Toxicity and developmental defects of different sizes and shape nickel nanoparticles in zebrafish. Sci. Technol. 2009; 43(16):6349-56. doi: 10.1021/es9010543
CrossRef - Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano letters. 2006 ;6(4):662-8. doi : 1021/nl052396o
CrossRef - Hamilton RF, Wu N, Porter D, Buford M, Wolfarth M, Holian A. Particle length-dependent titanium dioxide nanomaterials toxicity and bioactivity. Part Fibre Toxicol. 2009 ;6(1): 35. doi: 1186/1743-8977-6-35
CrossRef - Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Natl. Acad. Sci U S A. 2006 ;103(13):4930-4. doi : 10.1073/pnas.0600997103
CrossRef - Chen YS, Hung YC, Liau I, Huang GS. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res.2009; 858-64. doi: 10.1073/pnas.0600997103
CrossRef - Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004 ;15(4):897-900. doi : 1021/bc049951i
CrossRef - Wick P, Manser P, Limbach LK, et al. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett. 2007 ;168(2):121-31. doi: 1016/j.toxlet.2006.08.019
CrossRef - Warheit DB, Sayes CM. Routes of exposure to nanoparticles: hazard tests related to portal entries. In Nanoengineering 2015; 41-54. Elsevier.
CrossRef - Kreyling WG, Semmler-Behnke M, Takenaka S, Möller W. Differences in the biokinetics of inhaled nano-versus micrometer-sized particles. Chem. Res. 2013 ;46(3):714-22. doi: 10.1021/ar300043r.
CrossRef - Testing strategies to establish the safety of nanomaterials: conclusions of an ECETOC workshop. Inhal. Toxicol. 2007 ;19(8):631-43. doi: 1080/08958370701353080
CrossRef - Benn T, Cavanagh B, Hristovski K, Posner JD, Westerhoff P. The release of nanosilver from consumer products used in the home. Environ. Qual. 2010; 39(6):1875-82. doi: 10.2134/jeq2009.0363
CrossRef - Bergin IL, Witzmann FA. Nanoparticle toxicity by the gastrointestinal route: evidence and knowledge gaps. Int J Biomed Nanosci Nanotech. 2013 ;3(1-2):163-210. doi: 1504/IJBNN.2013.054515
CrossRef - Chen Z, Meng H, Xing G, et al. Acute toxicological effects of copper nanoparticles in vivo. Lett.,2006;163(2):109-20. Doi: 10.1016/j.toxlet.2005.10.003
CrossRef - Jani PU, Halbert GW, Langridge J, Florence AT. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol. 1990;42(12): 821- 6
CrossRef - Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005 ;113(7):823-39.doi: 1289/ehp.7339
CrossRef - Fabian E, Landsiedel R, Ma-Hock L, Wiench K, Wohlleben W, Van Ravenzwaay B. Tissue distribution and toxicity of intravenously administered titanium dioxide nanoparticles in rats. Toxicol. 2008; 82(3) :151-7. Doi:10.1007/s00204-007-0253-y
CrossRef - Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005 ;19(3):311-30.doi: 1096/fj.04-2747rev
CrossRef - Garnett MC, Kallinteri P. Nanomedicines and nanotoxicology: some physiological principles. Med. 2006 ;56(5):307-11. doi: 10.1093/occmed/kql052
CrossRef - Kohl Y, Rundén-Pran E, Mariussen E et al. Genotoxicity of nanomaterials: Advanced in vitro models and high throughput methods for human hazard assessment—A review. 2020 ;10(10):1911. doi: 3390/nano10101911
CrossRef - Pfuhler S, van Benthem J, Curren R, Doak SH, Dusinska M, Hayashi M, Heflich RH, Kidd D, Kirkland D, Luan Y, Ouedraogo G. Use of in vitro 3D tissue models in genotoxicity testing: Strategic fit, validation status and way forward. Report of the working group from the 7th International Workshop on Genotoxicity Testing (IWGT). Muta Res Gen Tox En. 2020 ;850:503135.doi :1016/j.mrgentox.2020.503135
CrossRef - Magdolenova Z, Drlickova M, Henjum K, et al. Coating-dependent induction of cytotoxicity and genotoxicity of iron oxide nanoparticles. 2015;9(sup1):44-56. doi: 10.3109/17435390.2013.847505
CrossRef - Genotoxicity of manufactured nanomaterials: report of the OECD expert meeting. Series on the Safety of Manufactured Nanomaterials No. 43. 2014.
- Doak SH, Dusinska M. NanoGenotoxicology: present and the future. Mutagenesis. 2017 ;32(1):1-4. doi: 1093/mutage/gew066
CrossRef - Evans SJ, Clift MJ, Singh N, et al. Critical review of the current and future challenges associated with advanced in vitro systems towards the study of nanoparticle (secondary) genotoxicity. Mutagenesis. 2017;32(1):233-41. doi: : 1093/mutage/gew054
CrossRef - Kohl Y, Rundén-Pran E, Mariussen E, et al. Genotoxicity of nanomaterials: Advanced in vitro models and high throughput methods for human hazard assessment—A review. Nanomaterials.2020 ;10(10):1911. doi : 3390/nano10101911
CrossRef - Wilde S, Dambowsky M, Hempt C, Sutter A, Queisser N. Classification of in vitro genotoxicants using a novel multiplexed biomarker assay compared to the flow cytometric micronucleus test. Environ Mol Mutagen.2017;58(9):662-77.doi: 1002/em.22130
CrossRef - Shah P, Kaushik A, Zhu X, Zhang C, Li CZ. Chip based single cell analysis for nanotoxicity assessment. Analyst. 2014;139(9):2088-98.
CrossRef - Hillegass JM, Shukla A, Lathrop SA, MacPherson MB, Fukagawa NK, Mossman BT. Assessing nanotoxicity in cells in vitro. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010 ;2(3):219-31. doi:1002/wnan.54
CrossRef - Hall PA, Levison DA. Review: assessment of cell proliferation in histological material. Clin. Pathol. 1990 ;43(3):184-92. doi: 10.1136/jcp.43.3.184
CrossRef - Coccini T, Pignatti P, Spinillo A, De Simone U. Developmental neurotoxicity screening for nanoparticles using neuron-like cells of human umbilical cord mesenchymal stem cells: example with magnetite nanoparticles. Nanomaterials. 2020;10(8):1607. doi: 3390/nano10081607
CrossRef - Sasaki T, Asakura M, Ishioka C, Kasai T, Katagiri T, Fukushima S. In vitro chromosomal aberrations induced by various shapes of multi‐walled carbon nanotubes (MWCNTs). J Occup Health. 2016 ;58(6):622-31. doi: 1539/joh.16-0099-OA
CrossRef - Huk A, Collins AR, El Yamani N et.al. Critical factors to be considered when testing nanomaterials for genotoxicity with the comet assay. Mutagenesis. 2015;30(1):85-8. doi:1093/mutage/geu077
CrossRef - Nandhakumar S, Parasuraman S, Shanmugam MM, Rao KR, Chand P, Bhat BV. Evaluation of DNA damage using single-cell gel electrophoresis (Comet Assay). J Pharmacol Pharmacother. 2011;2(2):107-11. doi: 4103/0976-500X.81903
CrossRef - Kain J, Karlsson HL, Möller L. DNA damage induced by micro-and nanoparticles—interaction with FPG influences the detection of DNA oxidation in the comet assay. 2012;27(4):491-500. doi:10.1093/mutage/ges010
CrossRef - Kim SC, Kim DW, Shim YH, et al. In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy. J Control Release. 2001;72(1-3):191-202. doi:1016/s0168-3659(01)00275-9
CrossRef - Lei R, Wu C, Yang B, et al. Integrated metabolomic analysis of the nano-sized copper particle-induced hepatotoxicity and nephrotoxicity in rats: a rapid in vivo screening method for nanotoxicity. Appl Pharmacol. 2008;232(2):292-301. Doi: 10.1016/j.taap.2008.06.026
CrossRef - Baker GL, Gupta A, Clark ML, Valenzuela BR, Staska LM, Harbo SJ, Pierce JT, Dill JA. Inhalation toxicity and lung toxicokinetics of C60 fullerene nanoparticles and microparticles. Sci. 2008;101(1):122-31. doi:10.1093/toxsci/kfm243
CrossRef - Jain J, Arora S, Rajwade JM, Omray P, Khandelwal S, Paknikar KM. Silver nanoparticles in therapeutics: development of an antimicrobial gel formulation for topical use. Mol. Pharmaceutics. 2009 Oct 5;6(5):1388-401. doi: 1021/mp900056g
CrossRef - Fiorito S, Serafino A, Andreola F, Bernier P. Effects of fullerenes and single-wall carbon nanotubes on murine and human macrophages. 2006 1;44(6):1100-5.
CrossRef - Yu T, Malugin A, Ghandehari H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano. 2011;5(7):5717-28. doi:1021/nn2013904
CrossRef - Karlsson HL. The comet assay in nanotoxicology research. Anal Bioanal Chem. 2010 ;398:651-66. doi:1007/s00216-010-3977-0
CrossRef - Gonzalez L, Sanderson BJ, Kirsch-Volders M. Adaptations of the in vitro MN assay for the genotoxicity assessment of nanomaterials. 2011;26(1):185-91. doi: 10.1093/mutage/geq088
CrossRef - Uo M, Tamura K, Sato Y, et al. The cytotoxicity of metal‐encapsulating carbon nanocapsules. 2005;1(8‐9):816-9.
CrossRef - Muller J, Huaux F, Moreau N et al. Respiratory toxicity of multi-wall carbon nanotubes. Applied Pharmacol. 2005;207(3):221-31.
CrossRef - Flahaut E, Durrieu MC, Remy-Zolghadri M, Bareille R, Baquey C. Investigation of the cytotoxicity of CCVD carbon nanotubes towards human umbilical vein endothelial cells. 2006;44(6):1093-9. doi:10.1016/j.carbon.2005.11.007
CrossRef - Sayes CM, Fortner JD, Guo W, et al. The differential cytotoxicity of water-soluble fullerenes. Nano Lett. 2004 ;4(10):1881-7.
CrossRef - Seleverstov O, Zabirnyk O, Zscharnack M, et al. Quantum dots for human mesenchymal stem cells labeling. A size-dependent autophagy activation. Nano Lett. 2006;6(12):2826-32. doi: 1021/nl0619711
CrossRef - Shvedova A, Castranova V, Kisin E, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health 2003;66(20):1909-26. doi:10.1080/713853956
CrossRef - Shukla RK, Sharma V, Pandey AK, Singh S, Sultana S, Dhawan A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol In Vitro. 2011 ;25(1):231-41. doi: 1016/j.tiv.2010.11.008
CrossRef - North M, Vulpe CD. Functional toxicogenomics: mechanism-centered toxicology. Int J Mol Sci. 2010 ;11(12):4796-813. Doi: 3390/ijms11124796
CrossRef - Lee HY, Choi YJ, Jung EJ, et al. Genomics-based screening of differentially expressed genes in the brains of mice exposed to silver nanoparticles via inhalation. Nanoparticle Res. 2010 ;12:1567-78. doi: 10.1007/s11051-009-9666-2
CrossRef - Jin X, Li M, Wang J, Marambio-Jones C, Peng F, Huang X, Damoiseaux R, Hoek EM. High-throughput screening of silver nanoparticle stability and bacterial inactivation in aquatic media: influence of specific ions. Environ Sci Technol. 2010 ;44(19):7321-8. doi: 1021/es100854g
CrossRef - Arora S, Rajwade JM, Paknikar KM. Nanotoxicology and in vitro studies: the need of the hour. Toxicol Applied Pharmacol. 2012 ;258(2):151-65. Doi: 1016/j.taap.2011.11.010
CrossRef - Tirumala MG, Anchi P, Raja S, Rachamalla M, Godugu C. Novel methods and approaches for safety evaluation of nanoparticle formulations: A focus towards in vitro models and adverse outcome pathways. Front Pharmacol. 2021;12:612659.doi: 3389/fphar.2021.612659
CrossRef - Buglak AA, Zherdev AV, Dzantiev BB. Nano-(Q) SAR for cytotoxicity prediction of engineered nanomaterials. Molecules. 2019 ;24(24):4537. doi: 3390/molecules24244537
CrossRef - Cramer RD, Patterson DE, Bunce JD. Comparative molecular field analysis (CoMFA). 1. Effect of shape on binding of steroids to carrier proteins. J Am Chem Soc. 1988 ;110(18):5959-67.doi: 1021/ja00226a005
CrossRef - Handral HK, Tong HJ, Islam I, Sriram G, Rosa V, Cao T. Pluripotent stem cells: An in vitro model for nanotoxicity assessments. J Appl Toxicol. 2016 ;36(10):1250-8. doi: 1002/jat.3347
CrossRef - Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. 2006;126(4):663-76. doi:10.1016/j.cell.2006.07.024
CrossRef - Fröhlich E. Role of omics techniques in the toxicity testing of nanoparticles. J Nanobiotechnology. 2017;15:1-22. doi: 1186/s12951-017-0320-3
CrossRef - Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine. 2012 :5577-91.doi:2147/IJN.S36111
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





