Manuscript accepted on : 01-05-2025
Published online on: 10-06-2025
Plagiarism Check: Yes
Reviewed by: Dr. Ozlem Coşkun
Second Review by: Dr. Ichrak Jaouadi
Final Approval by: Dr. Eugene A. Silow
Jaya Laxman Dolnar and Gautam Vithobaji Zodape*
and Gautam Vithobaji Zodape*
Department of Zoology, L.S. and S.S. Patkar College of Arts and Science and V. P. Varde College of Commerce and Economics, S.V. Road, Goregaon (West), Mumbai, Maharashtra, India.
Corresponding author Email : drgautamvz5@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3391
ABSTRACT: The research explores the biomedical properties of the box jellyfish Chiropsoides buitendijki, collected from Edwan village, Mumbai. The crude extract of the box jellyfish Chiropsoides buitendijki exhibits a range of bioactive properties with potential biomedical applications. Hemolytic activity observed in both human and chicken red blood cells indicates the presence of toxic proteins. The chick Chorio-Allantoic Membrane (CAM) assay revealed anti-angiogenic effects, including blood vessel damage and inhibition of neovascularization, suggesting potential use in cancer therapy. Neuromodulatory effects were assessed in Sprague-Dawley rat brains, where increased extract concentrations elevated Na⁺/K⁺-ATPase activity and cholinesterase inhibition, implicating potential impacts on metabolic stability and cognitive function. Protein analysis revealed a moderate protein content (0.121 mg/mL). Partial purification was achieved through SDS-PAGE electrophoresis, and the proteins were identified at various 11 kDa to 245 kDa levels, Functional characterization linked specific bands to hemolytic, antimicrobial, immunological, and anti-inflammatory activities. These findings underscore the pharmacological potential of Chiropsoides buitendijki extract, supporting its further exploration as a source of novel compounds for pharmaceutical and biomedical applications.
KEYWORDS: Anti-angiogenic; Cytotoxic; Hemolytic; Neuromodulatory; Proteins
Download this article as:| Copy the following to cite this article: Dolnar J. L, Zodape G. V. Biomedical Applications of Chiropsoides buitendijki Crude Extract: Exploring Hemolytic, Anti-Angiogenic, and Neuromodulatory Properties for Therapeutic Potential. Biotech Res Asia 2025;22(2). |
| Copy the following to cite this URL: Dolnar J. L, Zodape G. V. Biomedical Applications of Chiropsoides buitendijki Crude Extract: Exploring Hemolytic, Anti-Angiogenic, and Neuromodulatory Properties for Therapeutic Potential. Biotech Res Asia 2025;22(2). Available from: https://bit.ly/3FKanGc |
Introduction
The Dictionary of Marine Natural Products1 lists over 30,000 compounds from marine sources, with 1,200 new substances found each year.2 However, only about 5% of these come from Europe. The marine environment offers vast, untapped potential for discovering new pharmaceuticals.3 Over 80% of the world’s plant and animal life is found in marine environments. Marine organisms such as tunicates, sponges, soft corals, and sea slugs have proven to be rich sources of bioactive compounds.4 To date, researchers have identified approximately 10,000 different metabolites from these species, many of which show promising pharmacological potential.5
Jellyfish are invertebrates found in the ocean, with about 200 species in the Scyphozoa class.6 Some species, like Lobonema smithii and Rhopilema esculentum, are edible and rich in proteins, amino acids, carbohydrates, vitamins, and minerals.7,8 Box jellyfish, from the Cubozoa class, have cube-shaped bodies and contain venomous species, such as Chironex fleckeri, whose sting can be extremely painful, and potentially deadly.9 Their tentacles release venomous nematocysts, causing severe pain and tissue damage. 10, 11
Jellyfish venom contains proteins that play a crucial role in its toxic effects, leading to paralysis, pain, and other physiological responses in both prey and humans.12 While proteins are an essential component, their effects likely result from interactions with other biochemical compounds in the venom.13 Research on species like Rhopilemaa samushi and Cyanea nozakii has shown that jellyfish have high protein content, especially useful for collagen extraction, which is valuable in cosmetics and nutraceuticals.14 Many studies have focused on the neurotoxins found in Cnidarians, revealing the presence of potent neurotoxic proteins, such as those found in Carybdea marsupialis and Cotylorhiza tuberculata.15,16 Additionally, the cytolysin Rhizolysin, isolated from R. pulmo, has shown significant toxicity, with a molecular weight of 260 kDa.17
The venom of box jellyfish contains bioactive proteins that can cause hemolysis, cytotoxic effects, membrane damage, inflammation, cardiovascular failure, and even death in animals.18, 19 Research on the hemolytic effects of Cnidarians, including sea anemones, soft corals, scyphozoans, and cubozoans, showed that many species damage mammalian red blood cells. A study by20 found that all scyphozoan jellyfish tested showed hemolytic activity, with Trachymedusae species also containing hemolytically active compounds. Research by21 highlighted the growing interest in marine bioactive compounds for anti-angiogenesis over the past 30 years. A study by22 identified 43 marine compounds with anti-angiogenic properties, 10 of which are in clinical trials for cancer treatment.23 Found that marine compounds, including saccharides, terpenes, peptides, and alkaloids, show diverse structures and mechanisms that help fight cancer by targeting angiogenesis.
Tetrodotoxin, one of the most potent low-molecular-weight toxins, is synthesized by symbiotic bacteria and resides in specific species like puffers, ocean sunfishes, and porcupine fishes. It is notable for its ability to inhibit the normal increase in Na++ permeability without altering the movement of K+ outward and is currently undergoing Phase III trials for its potential use in treating neuropathic pain.24 The ATPase enzyme system is well-recognized for utilizing energy derived from ATP hydrolysis to actively transport sodium (Na⁺) and potassium (K⁺) ions across cell membranes.25 Research has demonstrated that the increase in metabolic activity caused by ionic transport at the membrane level triggers a cascade of biochemical reactions. This process results in the buildup of ADP and inorganic phosphate (Pi), both of which are crucial for the regulation of cellular respiration.
Elevated ADP levels boost mitochondria function, resulting in increased oxygen consumption and accelerated ATP production. Kossuga et al26 investigated tropical marine sponges and discovered their biological effects, including anti-acetyl cholinesterase activity. Additional studies have shown that the harmful effects of palytoxin on vulnerable organisms are associated with Na+ / K + -ATPase as a potential target at the molecular level.27 Research is ongoing to further elucidate the mechanisms of action of conotoxins. Despite increasing interest, the exploration of toxins as potential drug leads remains a relatively specialized area of research.
Materials and Methods
Samples collection
Specimens of the box jellyfish Chiropsoides buitendijki (Horst, R. 1907) were gathered during low tide from Edwan village, located on the west coast of Mumbai. The jellyfish were kept alive in seawater containers and taken to the lab. Once there, each jellyfish was rinsed twice with seawater and then once with distilled water. The cleaned samples were initially stored on ice and later transferred to a deep freezer maintained at -8°C for preservation, at the Department of Zoology, S.S. & L.S. Patkar College of Arts & Science, and V. P. Varde College of Commerce & Economics, Goregaon west, Mumbai.
Identification of box jellyfish
The initial identification of the jellyfish was based on their shape and the number of tentacles, along with a review of related scientific literature. Final confirmation of the species was provided by Dr. Ramkumar, a scientist at the Central Marine Fisheries Research Institute (CMFRI) in Mumbai.
Preparation of crude extract from box jellyfish
The crude extract of Chiropsoides buitendijki (Horst, R 1907) was prepared using a modified version of a method28 involving 80% methanol and 1% acetic acid. Ten grams of jellyfish tissue were blended, and 10 ml of a solution containing equal parts of 80% methanol and 1% acetic acid was added. The mixture was left to stand in a water bath at 45°C for 24 hours. After incubation, the solution was filtered using Whatman No.1 filter paper. The filtered homogenate was then centrifuged at 5000 rpm for 15 minutes at -8°C using a refrigerated centrifuge (Remi, Serial No. VCDX-5983). The resulting supernatant was collected in a conical flask and concentrated under reduced pressure using a rotary vacuum evaporator at 45°C. The concentrated extract was then passed through a Millipore filtration system, dried in a vacuum desiccator, and stored at -20°C until further analysis.
Ethical Approval
The collection of box jellyfish specimens for research was carried out with official permission from the Maharashtra State Biodiversity Board, Nagpur, under approval numbers MSBB/Desk-5/Research/841/2022-23 and MSBB/Desk-5/Research/397/2023-24. A reference sample of Chiropsoides buitendijki has been submitted to the Zoological Survey of India, Western Regional Centre, Pune, for preservation and is recorded under the accession number (ZSI-WRC Misc/19).
Hemolytic Activity
The micro hemolytic assay method was used to test the hemolytic activity of the crude extract of box jellyfish Chiropsoides buitendijki on human and chicken erythrocytes as proposed by.29
CAM (Chorio-Allantoic Membrane) Assay
The CAM (Chorio-Allantoic Membrane) assay, which uses the extra-embryonic membrane of chick embryos, was carried out with the crude extract of Chiropsoides buitendijki following the method described by.29
Neuromodulatory Activity
The assay of ATPase enzyme, the P2 fraction, or mitochondrial nerve terminals, was extracted from the brains of 20±2g female Sprague Dawley rats using29,30 procedure. The assay of acetylcholinesterase (AChE) activity was conducted by the procedure established by.30,31
Protein Estimation and SDS –PAGE Electrophoresis:
The protein content in the crude extract of Chiropsoides buitendijki was accurately determined using the Folin-Ciocalteau method, a well-established technique for protein quantification.32 Protein separation was then assessed through SDS-PAGE electrophoresis.
Results
Table 1: Demonstrating the hemolytic activity of the box jellyfish-Chiropsoides buitendijki extract the on human and chicken erythrocytes.
| Sr.No. | Blood | Protein of Crude extract(mg) | Total hemolysis (up to dilutions) | Hemolytic titer | Specific hemolytic activity(HT/mg) |
| 1 | Human | 0.121 | 10 | 56 | 462.80 |
| 2 | Chicken | 0.121 | 10 | 27 | 223.14 |
(Each analysis was achieved by five replicates)
Table 2: Demonstrating the effects of various concentrations of the box jellyfish (Chiropsoides buitendijki) extract on 9-day-old chicken eggs to evaluate its anti-angiogenic properties.
| Hours after treatment | Toxin Concentration in µg/ml | |||
| 20 | 40 | 60 | 80 | |
| 48 hrs. | + | + | ++ | +++ |
| 72 hrs. | + | + | +++ | +++ |
(+slight, ++Moderate, +++Severe)
Table 3: Demonstrating the effects of the box jellyfish-Chiropsoides buitendijki extract on rat brain Na+/ K+ ATPase activity
| Sr. No. | Concentration of Toxins (µg/mL) | Na+/ K+ ATPase activity(µPi/mg protein/hour) |
| 1 | 10µg/mL | 0.026 |
| 2 | 20µg/mL | 0.026 |
| 3 | 30µg/mL | 0.041 |
| 4 | 40µg/mL | 0.053 |
| 5 | 50µg/mL | 0.053 |
| 6 | 60µg/mL | 0.066 |
| 7 | 70µg/mL | 0.080 |
| 8 | 80µg/mL | 0.093 |
| 9 | 90µg/mL | 0.106 |
| 10 | 100 µg/mL | 0.106 |
(All results are average of triplicate sets)
Table 4: Demonstrating the effects of the box jellyfish-Chiropsoides buitendijki extract on rat brain AChE activity.
| Sr. No. | Concentration of crude extract(µg) | Level of Modulation (%) |
| 1 | 50 µg | 4.014 |
| 2 | 100 µg | 12.04 |
| 3 | 150 µg | 20.07 |
| 4 | 200µg | 32.11 |
| 5 | 500 µg | 36.13 |
(All results are average of triplicate sets)
Table 5: Showing estimation of protein from the crude extract of box jellyfish-Chiropsoides buitendijki by Folin-Ciocalteau method.
| Type of extract | OD of Standard | XOD ofextract | protein (mg/ml) at x660nm | Average concentration of xprotein(mg/ml) x |
| crude extract of box jellyfish-Chiropsoides buitendijki | 0.24 | 0.075 | 0.125 | 0.121 |
| 0.24 | 0.072x | 0.120 | ||
| 0.24 | 0.072 | 0.120 |
(Each analysis was achieved by five replicates)
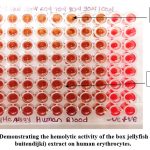 |
Photograph 1: Demonstrating the hemolytic activity of the box jellyfish (Chiropsoides buitendijki) extract on human erythrocytes. |
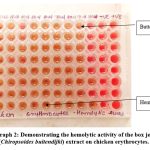 |
Photograph 2: Demonstrating the hemolytic activity of the box jellyfish (Chiropsoides buitendijki) extract on chicken erythrocytes. |
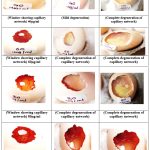 |
Photograph 3: Demonstrating the effects of the box jellyfish-Chiropsoides buitendijki extract on 9 days-old chicken egg to evaluate its anti-angiogenic properties |
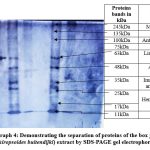 |
Photograph 4: Demonstrating the separation of proteins of the box jellyfish (Chiropsoides buitendijki) extract by SDS-PAGE gel electrophoresis |
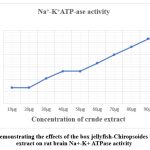 |
Graph 1: Demonstrating the effects of the box jellyfish-Chiropsoides buitendijki extract on rat brain Na+-K+ ATPase activity. |
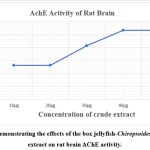 |
Graph 2: Demonstrating the effects of the box jellyfish-Chiropsoides buitendijki extract on rat brain AChE activity. |
Discussion
Hemolytic Activity
Jellyfish venom is known to produce a variety of biological effects, such as causing skin tissue damage (dermo-necrosis), affecting the nervous system (neurotoxicity), breaking down red blood cells (hemolysis), and impacting cardiovascular function.33,34 Chung et al35 isolated a potent hemolytic protein from the venom of Carybdea alata. Other species like Crambionellastu halmanni and Chrysaora quinquecirrha also contain peptides with biological activities, influenced by their molecular weight and amino acid sequences.36 Similarly, sponges, such as Halichondria panicea, have shown hemolytic activity, possibly due to lectins that cause hemagglutination.37 Studies on marine invertebrates indicate that 60% exhibit hemagglutinating or hemolytic activity.38,39 Additionally, Rhizostoma pulmo venom, when tested on human RBCs, showed minimal hemolytic effects.40
In our present study Table No. (1) Demonstrating the hemolytic activity of the box jellyfish-Chiropsoides buitendijki extract the on human and chicken erythrocytes and Photograph No. (1&2) Showing the hemolytic activity of the box jellyfish (Chiropsoides buitendijki) extract on human erythrocytes. The crude extract of Chiropsoides buitendijki (box jellyfish) demonstrated significant hemolytic activity in both human and chicken erythrocytes. In human blood, hemolysis occurred at concentrations between 50-100 µL, with a hemolytic titer of 56 and specific hemolytic activity of 462.80 (HT/mg). In chicken blood, hemolysis was observed between 40-100 µL, with a titer of 27 and specific activity of 223.14 (HT/mg). These results confirm that the crude extract contains potent toxins responsible for hemolytic activity, likely due to proteins in the extract.
CAM Assay
Marine bioactive compounds have gained attention for their anti-angiogenic properties, particularly in cancer research.21 Foundational work sparked interest, and recent studies have identified over 40 marine compounds with angiogenesis-inhibiting effects, with 10 currently in clinical trials.41 Many compounds including saccharides, terpenes, peptides, and alkaloids, offer a diverse mechanism for targeting angiogenesis in chick.42 Notable examples include aeroplysinin-1, isolated from Verongia aerophoba43 and Aplisina aerophoba,44 which show anticancer and anti-angiogenic properties. Other marine sponges, like Halichondria panacea29 and Agelas nakamurai,45 have also yielded compounds with promising anti-angiogenic activities.
In this study, Photograph No. (3) Demonstrating the effects of the box jellyfish-Chiropsoides buitendijki extract on 9 days-old chicken egg to evaluate its anti-angiogenic properties. In this study the crude extract of Chiropsoides buitendijki (box jellyfish) was tested using the chorio-allantoic membrane (CAM) assay. After 48 and 72 hours of incubation, different concentrations (20 µg/ml, 40 µg/ml, 60 µg/ml, 80 µg/ml) of the extract caused significant anti-angiogenic effects, including blood vessel lysis and disruption of new vessel formation. The extract primarily affected newly formed blood vessels, damaging preexisting vasculature. Compared to the positive control (Heparin), which showed typical branching blood vessel patterns, the extract caused severe blood vessel damage, suggesting its potential as a therapeutic agent for angiogenesis-related diseases, including cancer.
Neuromodulatory Activity
The ATPase enzyme system uses energy from ATP breakdown to transport Na+ and K+ ions. Recent studies show that tetrodotoxin (TTX), found in pufferfish, blocks nerve signal transmission and Na+ channel function by binding to Na+ channel-forming glycoproteins.46 Bacillus species and bile extracts from freshwater carps are recognized for their ability to inhibit the Na+/K+ ATPase system.47 Research on marine sponges reveals anti-acetylcholinesterase activity.48 Cholinesterase (ChE) breaks down acetylcholine (ACh) to stop nerve signals at cholinergic synapses. Inhibiting AChE treats conditions like Alzheimer’s, dementia, and Parkinson’s.49, 50 Alzheimer’s disease, affecting 25 million people globally, involves neurofibrillary tangles, amyloid plaques, and memory loss. Cholinesterase inhibitors like donepezil and rivastigmine help manage symptoms. Natural compounds, especially from marine organisms, are being explored for AChE inhibition to treat Alzheimer’s. Examples include polypeptides from mamba venom and alkaloids from Calabar beans. Marine species like Parazoanthus axinellae and jellyfish venom also show cholinesterase inhibitory activity.51
In our research, we used Sprague Dawley rats to study the effects of the box jellyfish-Chiropsoides buitendijki extract on rat brain Na+/ K+ ATPase and AChE activity. From the Table No. (3) and Graph No. (1) Demonstrating the effects of the box jellyfish-Chiropsoides buitendijki extract on rat brain Na+/ K+ ATPase activity. The extract was tested in vitro on rat brain tissue, with Na+/ K+ ATPase activity measured at doses ranging from 10 to 100 µg/mL. The highest activity (0.106 µPi/mg protein/hour) occurred at 100 µg/mL, while the lowest (0.026 µPi/mg protein/hour) was at 10 µg/mL. Results showed that Na+/ K+ ATPase activity increased with higher doses of the extract. From Table No. (4), and Graph No. (2) Demonstrating the effects of the box jellyfish-Chiropsoides buitendijki extract on rat brain AChE activity. The AChE activity increased with higher extract concentrations, reaching a peak of 36.13% at 500 µg/mL. The lowest activity (4.014%) was at 50 µg/mL. The research suggests that increased Na+/ K+ ATPase and AChE activity could contribute to metabolic complications and brain tissue damage. Cholinesterase enzymes, essential for breaking down acetylcholine, are important for memory and cognition.
Protein Estimation
Proteins in jellyfish venom play key roles in toxic effects such as paralysis and pain.52 While protein concentration in venom is moderate, proteins likely work in synergy with other compounds.53 For example, the protein content in S. fibulatus crude extracts was 1.6 mg/mL (chloroform) and 1.4 mg/mL (aqueous) protein content.54 Similarly, Callyspongia difusa had 1.62 mg/mL (methanol) and 1.43 mg/mL (aqueous) protein content.55 The protein content in sponge extracts like Haliclona molitba was much lower, with toxin-like proteins in bacterial isolates A03 and A03k at 2.78 g and 1.38 g, respectively. 56
Table No. (5) Showing estimation of protein from the crude extract of box jellyfish-Chiropsoides buitendijki by Folin-Ciocalteau method. The protein content of the box jellyfish (Chiropsoides buitendijki) extract was measured using the Folin-Ciocalteu method, yielding an average concentration of 0.121 mg/mL. This underscores the moderate protein presence in the extract and its relevance in jellyfish venom research.
SDS Page Electrophorises
A 120-kDa protein was successfully isolated from Carybdea marsupialis, a cubozoan jellyfish recognized for its strong neurotoxic impact on marine crabs Ocypode quadrata.57 This study supports the idea that large molecular weight proteins (like the 120 kDa protein) play a significant role in the neurotoxic mechanisms of jellyfish venom. Similarly,58 isolated smaller proteins (10-14 kDa) from Cotylorhiza tuberculata, a zooxanthellate jellyfish. These proteins likely contribute to the venom’s toxic effects, although Cotylorhiza tuberculata is classified as a non-dangerous jellyfish, highlighting how different species produce varying venom protein profiles with differing levels of toxicity. The discovery of Rhizolysin, a 260 kDa cytolysin from Rhopilema pulmo,59 and the identification of rhizoprotease (95 kDa) by,60 adds to our understanding of the diversity of venom components and their cytotoxic effects. This research underscores that venom from some species, even without the presence of nematocytes, can still exhibit cytotoxic and hemolytic activity, demonstrating the potency of certain venom proteins. This is particularly evident in the work by,61 which observed hemolysis and cytotoxicity in Rhopilema pulmo venom, supporting the idea that venom proteins themselves have intrinsic cytotoxic properties. The application of SDS-PAGE has been a key tool in identifying venom proteins across various species. For example,60 identified anticoagulant proteins in Aurelia sp. tentacles, with molecular weights ranging from 50 to 160 kDa, emphasizing the diversity of venom proteins in jellyfish. Hemolytic toxins in Carybdea alata were found at 42, 43, and 45 kDa, and similar bands were observed in Chrysaora achlyo.62 The identification of proteins in this range is significant because hemolytic activity is often associated with the disruption of red blood cells, which plays a central role in the venom’s overall toxic effects. Furthermore, gel filtration chromatography and other separation techniques have been instrumental in characterizing venom proteins from species like Carybdea marsupialis and Rhopilema nomadic, with protein peaks observed at 40-107 kDa.63 These findings indicate that venom proteins exhibit a wide range of molecular weights, which may be responsible for different biological activities, such as fibrinolysis, cytotoxicity, and anticoagulation. Studies of Nemopilema nomurai and Aurelia aurita by 64 demonstrated that venom proteins in the range of 60-80 kDa and 25-37 kDa have significant fibrinolytic activity. These findings align with previous research that demonstrated similar banding patterns across various jellyfish species, emphasizing that similar molecular weight protein may have conserved biological functions, such as fibrinolysis and hemolysis. In terms of sponge-derived toxins,30 identified several protein bands in the marine sponges Suberites carnosus and Sigmadocia fibulata using SDS-PAGE. Both sponges exhibited proteins in the 12-62 kDa range, with hemolytic activity linked to proteins at 20 kDa and 22 kDa. These proteins are likely responsible for the hemolytic effects observed, and the presence of lectins in both species suggests their potential role in immune modulation. This highlights the potential of sponge-derived toxins as sources of novel bioactive compounds.
In our present analysis photograph No. (4) Showing the separation of proteins of the crude extract of box jellyfish-Chiropsoides buitendijki by SDS-PAGE gel electrophoresis of the crude extract of Chiropsoides buitendijki (box jellyfish) revealed eight protein bands with molecular weights of 11 kDa, 17 kDa, 25 kDa, 35 kDa, 48 kDa, 63 kDa, 100 kDa, and 245 kDa. The 11 kDa proteins may disrupt Grb2-mediated signalling, while the 17 kDa and 25 kDa proteins showed hemolytic activity. The 35 kDa protein has biological and fibrinolytic activity, affecting immune cells and cellular bioactivity. The 48 kDa protein demonstrated antimicrobial properties against Pseudomonas aeruginosa and Candida albicans. The 63 kDa protein is involved in protein transport, and the 100 kDa protein is a heat shock protein with anti-inflammatory effects. The 245 kDa protein is considered a macro protein. Proteins in the 100-250 kDa range also exhibited hemolytic activity, confirming that the crude extract contains proteins with cytotoxic, hemolytic, hemagglutination, and neuromodulatory activities.
Conclusion
The findings confirm that the crude extract of the box jellyfish Chiropsoides buitendijki showed hemolytic activity on both human and chicken RBCs, confirming the presence of toxic proteins responsible for this effect. Additionally, the CAM assay on chicken eggs revealed anti-angiogenic properties, as the extract caused blood vessel damage and inhibited new vessel formation, suggesting potential applications in cancer therapy. The neuromodulatory effects of Chiropsoides buitendijki crude extract were assessed on Sprague-Dawley rat brains. Increased concentrations of the extract led to higher Na+/ K+ ATPase enzyme activity, potentially causing metabolic complications and brain tissue damage during seizures. The extract also exhibited increased cholinesterase enzyme inhibitor activity, which is linked to memory and cognitive functions, suggesting its potential as a source of acetylcholinesterase (AChE) inhibitors. Protein content analysis using the Folin-Ciocalteau method showed an average concentration of 0.121 mg/mL, highlighting the moderate protein presence in the extract. SDS-PAGE electrophoresis separated eight protein bands ranging from 11 kDa to 245 kDa. Notably, the 17 kDa and 25 kDa bands exhibited hemolytic activity, while the 35 kDa protein displayed biological effects related to immune response and fibrinolysis. The 48 kDa protein showed antimicrobial activity against Pseudomonas aeruginosa and Candida albicans. The 63 kDa proteins were linked to protein transport, and the 100 kDa band was identified as a heat shock protein involved in anti-inflammatory responses. The 245 kDa band is considered a macro protein. These results, directly supported by hemolysis assays, CAM assays, enzyme activity measurements, and protein profiling, confirm the presence of functionally diverse bioactive compounds. Notably, the detection of hemolytic proteins and cholinesterase inhibitors suggests specific therapeutic potential in areas such as targeted cell disruption and neurodegenerative disease treatment. The identification of anti-angiogenic and antimicrobial effects further underlines the biomedical relevance of this species. As one of the few studies to report such a comprehensive bioactivity profile from C. buitendijki, our findings emphasize the novel potential of jellyfish-derived compounds as a valuable source for future pharmaceutical and biomedical innovation. Further studies are needed to isolate and characterize the active components and elucidate their precise mechanisms of action.
Acknowledgement
Authors are thankful to Dr. Ramkumar, scientist, at the Central Marine Fisheries Research Institute (CMFRI), Mumbai for final identification and confirmation of species. Authors are also thankful to the Director, Maharashtra State Biodiversity Board, Nagpur, Maharashtra for giving permission for collection of species. Dr. Kishori Apte, National Toxicological Centre, APT Testing & Research Pvt. Ltd. Pune, Maharashtra for supervising and assisting during the toxicological studies.
Funding Sources
The authors are thankful to Mahatma Jyotiba Phule Research & Training Institute (MJPRF), Nagpur, Govt. of Maharashtra, for their financial support and cooperation for the research.
Conflict of Interest
The authors have no conflict of interest.
Data Availability Statement
This statement does not apply to this article.
Ethics Statement
Maharashtra State Biodiversity Board, Nagpur, under approval numbers MSBB/Desk-5/Research/841/2022-23 and MSBB/Desk-5/Research/397/2023-24 for the collection of box jellyfish specimens for research. National Toxicology Centre, Pune. The study was approved by the APT Foundation Ethical Committee (CPCSEA RP. No APTRF/RP-02/2223).
Informed Consent Statement
This study did not involve human participants, and therefore, informed consent was not required.
Clinical Trial Registration
This research does not involve any clinical trials.
Permission to reproduce material from other sources
Not Applicable.
Author Contributions
Jaya laxman Dolnar: Data collection, analysis & writing;
Gautam Vithobaji Zodape: Conceptualization and supervision
References
- Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR. Marine natural products. Nat Prod Rep.2016 Mar;33(3):382-431. doi: 10.1039/c5np00156k. Epub 2016 Feb 3. PMID: 26837534.
CrossRef - Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR. Marine natural products. Nat Prod Rep.2012 Feb;29(2):144-222. doi: 10.1039/c2np00090c. Epub 2011 Dec 22. PMID: 22193773
CrossRef - McCarthy, P. J., & Pomponi, S. A. A search for new pharmaceutical drugs from marine organisms. Marine Biomedical Research.2004:1-2.
CrossRef - Donia M, Hamann MT. Marine natural products and their potential applications as anti-infective agents. Lancet Infect Dis.2003Jun;3(6):338-48. doi:10.1016/s1473-3099(03)00655-8. PMID: 12781505; PMCID: PMC7106398.
CrossRef - Faulkner, D. Biomedical uses for natural marine chemicals. Oceanus.1995;35:29–35.
- Nishimoto, S., Goto, Y., Morishige, H., Shiraishi, R., Doi, M., Akiyama, K., Yamauchi, S., & Sugahara, T. Mode of action of the immunostimulatory effect of collagen from jellyfish. Bioscience, Biotechnology, and Biochemistry.2008;72:2806–2814. doi: 10.1271/bbb.80154. Epub 2008 Nov 7.
CrossRef - Li, Q.-M., Wang, J.-F., Zha, X.-Q., Pan, L.-H., Zang, H.-L., & Luo, J. Structural characterization and immunomodulatory activity of new polysaccharides from jellyfish. Carbohydrate Polymers.2017; 159:188–194. doi: 10.1016/j.carbpol.2016.12.031. Epub 2016 Dec 16
CrossRef - Cao Y, Yisimayi A, Bai Y, Huang W, Li X, Zhang Z. et al. Humoral immune response to circulating SARS-CoV-2 variants elicited by inactivated and RBD-subunit vaccines. Cell Research.2021Jul;31(7):732-741. doi: 10.1038/s41422-021-00514-9. Epub 2021 May 21.
CrossRef - Endean, R., & Sizemore, D. J. The effectiveness of antivenom in countering the actions of box-jellyfish (Chironex fleckeri) nematocyst toxins in mice. 1988;26(5):425-31. doi: 10.1016/0041-0101(88)90181-x. PMID: 2903586.
CrossRef - Currie BJ, Jacups SP. Prospective study of Chironex fleckeri and other box jellyfish stings in the “Top End” of Australia’s Northern Territory. Med J Aust.2005 Dec 5-19;183(11-12):631-6. doi: 10.5694/j.1326-5377. 2005.tb00062. x. PMID: 16336157.
CrossRef - Lumley J, Williamson JA, Fenner PJ, Burnett JW, Colquhoun DM. Fatal envenomation by Chironex fleckeri, the north Australian box jellyfish: the continuing search for lethal mechanisms. Med J Aust.1988 May 16;148(10): 527-34.doi: 10.5694/j.1326-5377. 1988.tb99466. x. PMID: 2897074.
CrossRef - Carrette T, Seymour J. A rapid and repeatable method for venom extraction from cubozoan nematocysts. 2004 Aug;44(2): 135-9.doi: 10.1016/j.toxicon.2004.04.008. PMID: 15246760.
CrossRef - Klint, J.K., Senff, S., Rupasinghe, D.B., Er, S.Y., Herzig, V., Nicholson, G.M., King, G.F. Spider-venom peptides that target voltage-gated sodium channels: pharmacological tools and potential therapeutic leads. Toxicon.2012;60(4):478–491.doi: 10.1016/j.toxicon.2012.04.337
CrossRef - Leone A, Lecci RM, Durante M, Meli F, Piraino S. The Bright Side of Gelatinous Blooms: Nutraceutical Value and Antioxidant Properties of Three Mediterranean Jellyfish (Scyphozoa). Mar Drugs. 2015 Jul 29;13(8): 4654-81.doi: 10.3390/md13084654. PMID: 26230703; PMCID: PMC4556998.
CrossRef - Sanchez-Rodriguez J, Torrens E, Segura-Puertas L. Partial purification and characterization of a novel neurotoxin and three cytolysins from box jellyfish (Carybdea marsupialis) nematocyst venom. Arch Toxicol. 2006 Mar;80(3): 163-8.doi: 10.1007/s00204-005-0023-7. Epub 2005 Nov 8. PMID: 16283253.
CrossRef - Leone A, Lecci RM, Durante M, Piraino S. Extract from the zooxanthellate jellyfish Cotylorhiza tuberculata modulates gap junction intercellular communication in human cell cultures. Mar Drugs. 2013 May 22;11(5): 1728-62.doi: 10.3390/md11051728. PMID: 23697954; PMCID: PMC3707171.
CrossRef - Cariello L, de Santis A, Fiore F, Piccoli R, Spagnuolo A, Zanetti L, Parente A. Calitoxin, a neurotoxic peptide from the sea anemone Calliactis parasitica: amino acid sequence and electrophysiological properties. Biochemistry. 1989 Mar 21;28(6):2484-9. doi: 10.1021/bi00432a020. PMID: 2567180.
CrossRef - Badre, S. Bioactive toxins from stinging jellyfish. Toxicon. 2014 Dec; 91:114-25. doi: 10.1016/j.toxicon.2014.09.010. Epub 2014 Oct 5. PMID: 25286397.
CrossRef - Brinkman DL, Burnell JN. Biochemical and molecular characterisation of cubozoan protein toxins. 2009 Dec 15;54(8): 1162-73.doi: 10.1016/j.toxicon.2009.02.006. Epub 2009 Feb 20. PMID: 19232527.
CrossRef - Takenori, K., Lindsay, D. J., Kitamura, M., Konishi, S., Nishikawa, J., Nishida, S., Kamio, M., & Nagai, H. Evaluation of the bioactivities of water-soluble extracts from twelve deep-sea jellyfish species. Fisheries Science, 2013;79:487–494.doi 10.1007/s12562-013-0612-y
CrossRef - Folkman J. Tumour angiogenesis: Therapeutic implications. N Engl J Med. 1971; 285:1182–1186. doi: 10.1056/NEJM197111182852108.
CrossRef - Wang YQ, Miao ZH. Marine-derived angiogenesis inhibitors for cancer therapy. Mar Drugs. 2013 Mar 15;11(3):903-33. doi: 10.3390/md11030903. PMID: 23502698; PMCID: PMC3705379.
CrossRef - Nastrucci C, Cesario A, Russo P. Anticancer drug discovery from the marine environment. Recent Pat Anticancer Drug Discov. 2012 May 1;7(2):218-32. doi: 10.2174/157489212799972963. PMID: 22216781.
CrossRef - Nieto FR, Cobos EJ, Tejada MÁ, Sánchez-Fernández C, González-Cano R, Cendan CM. Tetrodotoxin (TTX) as a therapeutic agent for pain. Mar Drugs. 2012 Feb;10(2):281-305. doi: 10.3390/md10020281. Epub 2012 Jan 31. PMID: 22412801; PMCID: PMC3296997.
CrossRef - Lehninger, A.L. The mitochondrian. New York, Amsterdam: A. Benjamin.1964;132.
- Kossuga MH, Nascimento AM, Reimão JQ. et al. Antiparasitic, anti-neuroinflammatory, and cytotoxic polyketides from the marine sponge Plakortis angulospiculatus collected in Brazil. J Nat Prod. 2008 Mar;71(3):334-9. doi: 10.1021/np0705256. Epub 2008 Jan 5. PMID: 18177008.
CrossRef - Sanchez-Rodríguez J, Cruz-Vazquez K. Isolation and biological characterization of neurotoxic compounds from the sea anemone Lebrunia danae (Duchassaing and Michelotti, 1860). Arch Toxicol. 2006 Jul;80(7):436-41. doi: 10.1007/s00204-006-0059-3. Epub 2006 Feb 11. PMID: 16474963
CrossRef - Braekman, J. C., Daloze, D., Stoller, C., & van Soest, R. W. M. Chemotaxonomy of Agelas (Porifera: Demospongiae). Biochemical Systematics and Ecology.1992; 20:417–431. ISSN 0305-1978, https:// doi.org/10.1016/0305-1978(92)90082-O.
CrossRef - Purushottama, G. B., Venkateshvaran, K., Pani Prasad, K., & Nalini, P. Bioactivities of extracts from the marine sponge Halichondria panicea. Venomous Animals and Toxins Including Tropical Diseases.2009;15(3):444-459. doi:10.1590/S167891992009000300007
CrossRef - Zodape GV & Bhadekar NS. Biomedical Activities of Marine Sponge Suberites carnosus (Johnston) Collected from West Coast of Mumbai, India. Saudi J Med Pharm Sci. 2021;7(7): 307-319. doi: 10.36348/sjmps. 2021.v07i07.005
CrossRef - Ellithey MS, Lall N, Hussein AA, Meyer D. Cytotoxic and HIV-1 enzyme inhibitory activities of Red Sea marine organisms. BMC Complement Altern Med. 2014 Feb 25; 14:77. doi: 10.1186/1472-6882-14-77. PMID: 24568567; PMCID: PMC3939812.
CrossRef - Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265-75. PMID: 14907713.
CrossRef - Tibballs J. Australian venomous jellyfish, envenomation syndromes, toxins and therapy. Toxicon. 2006 Dec 1;48(7):830-59. doi: 10.1016/j.toxicon.2006.07.020. Epub 2006 Jul 15. PMID: 16928389.
CrossRef - Suput D. In vivo effects of cnidarian toxins and venoms. Toxicon. 2009 Dec 15;54(8):1190-200. doi: 10.1016/j.toxicon.2009.03.001. Epub 2009 Mar 10. PMID: 19281834.
CrossRef - Chung JJ, Ratnapala LA, Cooke IM, Yanagihara AA. Partial purification and characterization of a hemolysin (CAH1) from Hawaiian box jellyfish (Carybdea alata) Toxicon. 2001 Jul;39(7):981-90. doi: 10.1016/s0041-0101(00)00237-3. PMID: 11223087.
CrossRef - Suganthi, K., Bragadeeswaran, S., Sri Kumaran, N., Thangaraj, S., & Balasubramanian, T. Biological and pharmacological activities of jellyfish Crambionella stuhalmanni (Chun, 1896) and Chrysaora quinquecirrha (Desor, 1843). International Journal of Pharmaceutical Sciences. 2011; 3(2), 230–236.
- Bhadekar, N. S., &Zodape, G. V. Effect of marine sponge Sigmadociafibulata (Schmidt) and Suberites carnosus (Johnston) on histopathological examination of liver, kidney, heart, lung, and brain tissues of female Sprague-Dawley rats. Saudi Journal of Medical and Pharmaceutical Sciences.2020;6(3):281–290. doi:10.36348/sjmps. 2020.v06i03.005
CrossRef - Dresch, R. R., Haeser, A. S., Lerner, C., Mothes, B., Vozari-Hampe, M., & Henriques, A. T. Detection of lectinic activity and hemolytic activity in sponge strata (Porífera) native to the Atlantic coast of Brazil. Brazilian Journal of Pharmacognosy, 2005; 15(1), 16–22.
CrossRef - Mojica, E., Deocaris, C. C., &Merca, F. E. A survey of lectin-like activity in Philippine marine invertebrates. Philippine Journal of Science.2005; 134(2):135–142.doi-https://www.researchgate.net/publication/264417320
- Allavena A, Mariottini GL, Carli AM, Contini S, Martelli A. In vitro evaluation of the cytotoxic, hemolytic and clastogenic activities of Rhizostoma pulmo toxin(s). Toxicon. 1998 Jun;36(6):933-6. doi: 10.1016/s0041-0101(97)00171-2. PMID: 9663699.
CrossRef - Wang YQ, Miao ZH. Marine-derived angiogenesis inhibitors for cancer therapy. Mar Drugs. 2013 Mar 15;11(3):903-33. doi: 10.3390/md11030903. PMID: 23502698; PMCID: PMC3705379.
CrossRef - Nastrucci, C., Cesario, A., & Russo, P. Anticancer drug discovery from the marine environment. Recent Patents on Anti-Cancer Drug Discovery. 2012; 7, 218–232. doi: 10.2174/157489212799972963
CrossRef. - Martínez-Poveda B, Rodríguez-Nieto S, García-Caballero M, Medina MÁ, Quesada AR. The antiangiogenic compound aeroplysinin-1 induces apoptosis in endothelial cells by activating the mitochondrial pathway. Mar Drugs. 2012 Sep;10(9):2033-2046. doi:10.3390/md10092033. Epub 2012 Sep 18. PMID: 23118719; PMCID: PMC3475271.
CrossRef - Rodríguez-Nieto S, González-Iriarte M, Carmona R, Muñoz-Chápuli R, Medina MA, Quesada AR. Antiangiogenic activity of aeroplysinin-1, a brominated compound isolated from a marine sponge. FASEB J. 2002 Feb;16(2):261-3. doi: 10.1096/fj.01-0427fje. Epub 2001 Dec 28. PMID: 11772945.
CrossRef - Thomas TR, Kavlekar DP, LokaBharathi PA. Marine drugs from sponge-microbe association–a review. Mar Drugs. 2010 Apr 22;8(4):1417-6 8.doi: 10.3390/md8041417. PMID: 20479984; PMCID: PMC2866492.
CrossRef - Narahashi, T., Moore, J. W., & Scott, W. R. Tetrodotoxin blockage of sodium conductance increases in lobster giant axons. Journal of General Physiology,1964; 47(5), 965–974.
CrossRef - Wankhede, M. Neuroinhibitory activity of fish bile and ovarian extracts of the horseshoe crab (Dissertation). Central Institute of Fisheries Education, Mumbai. 1996.
- Kossuga MH, Nascimento AM, Reimão JQ, Tempone AG, Taniwaki NN, Veloso K, Ferreira AG, Cavalcanti BC, Pessoa C, Moraes MO, Mayer AM, Hajdu E, Berlinck RG. Antiparasitic, anti neuro inflammatory, and cytotoxic polyketides from the marine sponge Plakortis angulospiculatus collected in Brazil. J Nat Prod. 2008 Mar;71(3):334-9. doi: 10.1021/np0705256. Epub 2008 Jan 5. PMID: 18177008.
CrossRef - Atta-ur Rahman, & Choudhary, M. Bioactive natural products as a potential source of new pharmacophores: A theory of memory. Pure and Applied Chemistry. 2001; Vol.73 (3):555-560. doi-http://dx.doi.org/10.1351/pac200173030555
CrossRef - Karlsson E, Mbugua PM, Rodriguez-Ithurralde D. Fasciculins, anticholinesterase toxins from the venom of the green mamba Dendroaspis angustice J Physiol (Paris). 1984;79(4):232-40. PMID: 6530667.
- Yosra, A., Ayed, A., Dellai, A., Ben Mansour, H., Bacha, H., & Abid, S. Analgesic and anti-butyrylcholinesterase activities of the venom prepared from the Mediterranean jellyfish Pelagia noctiluca (Forsskal, 1775). Annals of Clinical Microbiology and Antimicrobials.2012 Jun 12:11-15. doi: 10.1186/1476-0711-11-15. PMID: 22691546; PMCID: PMC3483011.
CrossRef - Carrette, T., & Seymour, J. A rapid and repeatable method for venom extraction from cubozoan nematocysts. Toxicon, 2004; 44 (2), 135–139. doi: 10.1016/j.toxicon.2004.04.008.
CrossRef - Klint, J. K., Smith, J. J., Vetter, I., Rupasinghe, D. B., Er, S. Y., Senff, S., & King, G. F. Production of a selective Na V 1.7 inhibitor from centipede venom by recombinant expression in Escherichia coli. PLOS ONE, 2012; 7(10): e47938.
- Richet, C. De la variability de la dose toxique de suberitine. C R Seances Acad Sci (III). 1965; 61:686–688.
- Boobathy, S., Soundarapandian, P., Subasri, V., Vembu, N., & Gunasundari, V. Bioactivities of protein isolated from marine sponge Sigmadocia fibulatus. Current Research Journal of Biological Sciences.2009; 1(3):160–162.
- Vivitri, D., Prasasty, R. R., & Rory, A. HutagalungStructure-function analysis of toxin-like protein of Haliclonamolitba associated with symbiotic bacteria from Indonesian islands. IJSRSET, 2017 Jan;1(3):01–05. IJSRSET1626156
- Sánchez-Rodríguez J, Torrens E, Segura-Puertas L. Partial purification and characterization of a novel neurotoxin and three cytolysins from box jellyfish (Carybdea marsupialis) nematocyst venom. Arch Toxicol. 2006 Mar;80(3):163-8. doi: 10.1007/s00204-005-0023-7. Epub 2005 Nov 8. PMID: 16283253.
CrossRef - Leone, A., Lecci, R. M., Durante, M., Piraino, S. Extract from the zooxanthellate jellyfish Cotylorhiza tuberculata modulates gap junction intercellular communication in human cell cultures. Marine Drugs, 2013;11(5):1728–1762. doi: 3390/md11051728
CrossRef - Cariello, L., de Santis, A., Fiore, F., Piccoli, R., Spagnuolo, A., Zanetti, L., & Parente, A. Calitoxin: A neurotoxic peptide from the sea anemone Calliactis parasitica: Amino acid sequence and electrophysiological properties. 1989 Mar 21;28(6):2484-9. doi: 10.1021/bi00432a020. PMID: 2567180.
CrossRef - Rastogi Akriti, Sumit Biswas, Angshuman Sarkar, Dibakar Chakrabarty. Anticoagulant activity of Moon jellyfish (Aurelia aurita) tentacle extract. 2012 Oct;60(5):719-23. doi: 10.1016/j.toxicon.2012.05.008.
CrossRef - Allavena, A., Mariottini, G. L., Carli, A. M., & Contini, S. In vitro evaluation of the cytotoxic, hemolytic and clastogenic activities of Rhizostoma pulmo Toxicon.1998 Jun;36(6):933-6. doi: 10.1016/s0041-0101(97)00171-2.
CrossRef - Radwan, F. F., Gershwin, L. A., & Burnett, J. W. Toxinological studies on the nematocyst venom of Chrysaora achlyos. Toxicon. 2000 Nov;38(11):1581-91. doi: 10.1016/s0041-0101(00)00092-1.
CrossRef - Gusmani, L., Avian, M., Galil, B., Patriarca, P., & Rottini, G. Biologically active polypeptides in the venom of the jellyfish Rhopilema nomadic. Toxicon. 1997 May;35(5): 637-48. doi: 10.1016/s0041-0101(96)00182-1.
CrossRef - Lee H, Bae SK, Kim M, Pyo MJ, Kim M, Yang S, Won CK, Yoon WD, Han CH, Kang C, Kim E. Anticancer Effect of Nemopilema nomuraiJellyfish Venom on HepG2 Cells and a Tumor Xenograft Animal Model. Evid Based Complement Alternat Med. 2017; 2017:2752716. doi: 10.1155/2017/2752716. Epub 2017 Jul 13. PMID: 28785288; PMCID: PMC5530421.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





